Ultrasensitive Boron-Nitrogen-Codoped CVD Graphene-Derived NO2 Gas Sensor
- PMID: 36855380
- PMCID: PMC9888635
- DOI: 10.1021/acsmaterialsau.2c00003
Ultrasensitive Boron-Nitrogen-Codoped CVD Graphene-Derived NO2 Gas Sensor
Abstract
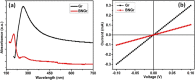
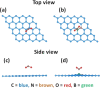
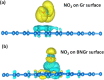
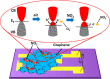
Recent trends in 2D materials like graphene are focused on heteroatom doping in a hexagonal honeycomb lattice to tailor the desired properties for various lightweight atomic thin-layer derived portable devices, particularly in the field of gas sensors. To design such gas sensors, it is important to either discover new materials with enhanced properties or tailor the properties of existing materials via doping. Herein, we exploit the concept of codoping of heteroatoms in graphene for more improvements in gas sensing properties and demonstrate a boron- and nitrogen-codoped bilayer graphene-derived gas sensor for enhanced nitrogen dioxide (NO2) gas sensing applications, which may possibly be another alternative for an efficient sensing device. A well-known method of low-pressure chemical vapor deposition (LPCVD) is employed for synthesizing the boron- and nitrogen-codoped bilayer graphene (BNGr). To validate the successful synthesis of BNGr, the Raman, XPS, and FESEM characterization techniques were performed. The Raman spectroscopy results validate the synthesis of graphene nanosheets, and moreover, the FESEM and XPS characterization confirms the codoping of nitrogen and boron in the graphene matrix. The gas sensing device was fabricated on a Si/SiO2 substrate with prepatterned gold electrodes. The proposed BNGr sensor unveils an ultrasensitive nature for NO2 at room temperature. A plausible NO2 gas sensing mechanism is explored via a comparative study of the experimental results through the density functional theory (DFT) calculations of the adsorbed gas molecules on doped heteroatom sites. Henceforth, the obtained results of NO2 sensing with the BNGr gas sensor offer new prospects for designing next-generation lightweight and ultrasensitive gas sensing devices.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Duy L. T.; Kim D. J.; Trung T. Q.; Dang V. Q.; Kim B. Y.; Moon H. K.; Lee N. E. High performance three-dimensional chemical sensor platform using reduced graphene oxide formed on high aspect-ratio micro-pillars. Adv. Funct. Mater. 2015, 25, 883–890. 10.1002/adfm.201401992. - DOI
LinkOut - more resources
Full Text Sources
