Immunoglobulin, glucocorticoid, or combination therapy for multisystem inflammatory syndrome in children: a propensity-weighted cohort study
- PMID: 36855438
- PMCID: PMC9949883
- DOI: 10.1016/S2665-9913(23)00029-2
Immunoglobulin, glucocorticoid, or combination therapy for multisystem inflammatory syndrome in children: a propensity-weighted cohort study
Abstract
Background: Multisystem inflammatory syndrome in children (MIS-C), a hyperinflammatory condition associated with SARS-CoV-2 infection, has emerged as a serious illness in children worldwide. Immunoglobulin or glucocorticoids, or both, are currently recommended treatments.
Methods: The Best Available Treatment Study evaluated immunomodulatory treatments for MIS-C in an international observational cohort. Analysis of the first 614 patients was previously reported. In this propensity-weighted cohort study, clinical and outcome data from children with suspected or proven MIS-C were collected onto a web-based Research Electronic Data Capture database. After excluding neonates and incomplete or duplicate records, inverse probability weighting was used to compare primary treatments with intravenous immunoglobulin, intravenous immunoglobulin plus glucocorticoids, or glucocorticoids alone, using intravenous immunoglobulin as the reference treatment. Primary outcomes were a composite of inotropic or ventilator support from the second day after treatment initiation, or death, and time to improvement on an ordinal clinical severity scale. Secondary outcomes included treatment escalation, clinical deterioration, fever, and coronary artery aneurysm occurrence and resolution. This study is registered with the ISRCTN registry, ISRCTN69546370.
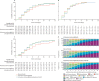
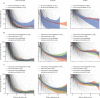
Findings: We enrolled 2101 children (aged 0 months to 19 years) with clinically diagnosed MIS-C from 39 countries between June 14, 2020, and April 25, 2022, and, following exclusions, 2009 patients were included for analysis (median age 8·0 years [IQR 4·2-11·4], 1191 [59·3%] male and 818 [40·7%] female, and 825 [41·1%] White). 680 (33·8%) patients received primary treatment with intravenous immunoglobulin, 698 (34·7%) with intravenous immunoglobulin plus glucocorticoids, 487 (24·2%) with glucocorticoids alone; 59 (2·9%) patients received other combinations, including biologicals, and 85 (4·2%) patients received no immunomodulators. There were no significant differences between treatments for primary outcomes for the 1586 patients with complete baseline and outcome data that were considered for primary analysis. Adjusted odds ratios for ventilation, inotropic support, or death were 1·09 (95% CI 0·75-1·58; corrected p value=1·00) for intravenous immunoglobulin plus glucocorticoids and 0·93 (0·58-1·47; corrected p value=1·00) for glucocorticoids alone, versus intravenous immunoglobulin alone. Adjusted average hazard ratios for time to improvement were 1·04 (95% CI 0·91-1·20; corrected p value=1·00) for intravenous immunoglobulin plus glucocorticoids, and 0·84 (0·70-1·00; corrected p value=0·22) for glucocorticoids alone, versus intravenous immunoglobulin alone. Treatment escalation was less frequent for intravenous immunoglobulin plus glucocorticoids (OR 0·15 [95% CI 0·11-0·20]; p<0·0001) and glucocorticoids alone (0·68 [0·50-0·93]; p=0·014) versus intravenous immunoglobulin alone. Persistent fever (from day 2 onward) was less common with intravenous immunoglobulin plus glucocorticoids compared with either intravenous immunoglobulin alone (OR 0·50 [95% CI 0·38-0·67]; p<0·0001) or glucocorticoids alone (0·63 [0·45-0·88]; p=0·0058). Coronary artery aneurysm occurrence and resolution did not differ significantly between treatment groups.
Interpretation: Recovery rates, including occurrence and resolution of coronary artery aneurysms, were similar for primary treatment with intravenous immunoglobulin when compared to glucocorticoids or intravenous immunoglobulin plus glucocorticoids. Initial treatment with glucocorticoids appears to be a safe alternative to immunoglobulin or combined therapy, and might be advantageous in view of the cost and limited availability of intravenous immunoglobulin in many countries.
Funding: Imperial College London, the European Union's Horizon 2020, Wellcome Trust, the Medical Research Foundation, UK National Institute for Health and Care Research, and National Institutes of Health.
© 2023 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license.
Conflict of interest statement
AT has provided unpaid consultancy work for Janssen Pharmaceuticals. DM has received grant support from the British Embassy in Moscow (StopCOVID Cohort: Clinical Characterisation of Russian Patients) and from UK Research and Innovation/National Institute for Health and Care Research (NIHR; Long COVID Core Outcome Set [PC-COS] project), and holds the following unpaid positions: Co-Chair of International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC) Global Paediatric Long COVID Working Group, Member of ISARIC working group on long-term follow-up in adults, Co-lead of the PC-COS project aiming to define the Core Outcome Set for Long-COVID, in collaboration with the WHO. MJC reports a personal fee from Biotest for speaking at the BioTest Immunology Forum 2022, Royal Society. EW holds the following unpaid positions: member of the paediatric steering committee for the RECOVERY trial; Paediatric Representative for NHS England working on the National paediatric virtual advisory network and expert advisory group for COVID treatment, and independent advisory group for COVID monoclonal antibodies; and Co-lead for the pan-London Post-COVID service for children. All other authors declare no competing interests.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

