Recent Advances in Light-Induced Selenylation
- PMID: 36855533
- PMCID: PMC9955339
- DOI: 10.1021/acsorginorgau.2c00033
Recent Advances in Light-Induced Selenylation
Abstract
Selenium-containing organic molecules have recently found a plethora of applications, ranging from organic synthesis to pharmacology and material sciences. In view of these concepts, the development of mild, efficient, and general protocols for the formation of C-Se bonds is desirable, and light induced approaches are appealing ways. The aim of this Review is to provide the reader with the most recent examples of light promoted selenylation processes.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

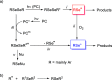
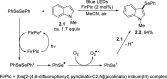
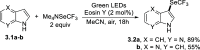

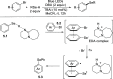

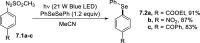
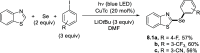
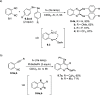



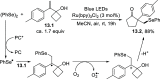


References
-
- Makhal P. N.; Nandi A.; Kaki V. R. Insights into the Recent Synthetic Advances of Organoselenium Compounds. ChemistrySelect 2021, 6, 663–679. 10.1002/slct.202004029. - DOI
-
- Lenardão E. J.; Santi C.; Sancineto L.. New Frontiers in Organoselenium Compounds; Springer International Publishing: Cham, Switzerland, 2018.
-
- Panda A. Chemistry of Selena macrocycles. Coord. Chem. Rev. 2009, 253, 1056–1098. 10.1016/j.ccr.2008.07.006. - DOI
Publication types
LinkOut - more resources
Full Text Sources
