Solubilisation and Enhanced Oral Absorption of Curcumin Using a Natural Non-Nutritive Sweetener Mogroside V
- PMID: 36855540
- PMCID: PMC9968502
- DOI: 10.2147/IJN.S395266
Solubilisation and Enhanced Oral Absorption of Curcumin Using a Natural Non-Nutritive Sweetener Mogroside V
Abstract
Background: Curcumin (CUR) is a functional ingredient from the spice turmeric. It has attracted considerable attention recently, owing to its diverse biological activities. However, curcumin has low water solubility, which limited its applications. Some sugar molecules were found to be able to solubilise poorly water-soluble compounds by forming micelles in aqueous solutions.
Purpose: To improve the water solubility and oral absorption of CUR, using a non-nutritive natural sweetener, namely, Mogroside V (Mog-V).
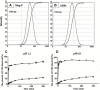
Methods: A solid dispersion of CUR in Mog-V was prepared using a solvent evaporation method. The solid dispersion was characterised by using X-ray diffraction and differential scanning calorimetry. The solid dispersion can dissolve in water to form micelles with a diameter of ~160 nm, which were characterised by using dynamic light scattering. To find out the mechanism of solubilisation, the aggregation behaviour of Mog-V molecules in aqueous solution was investigated using nuclear magnetic resonance spectroscopy. Finally, oral absorption of CUR in the solid dispersion was evaluated using a rodent model.
Results: A solid dispersion was formed in a ratio of 1 CUR to 10 Mog-V by weight. Upon dissolution into water, CUR laden micelles formed via self-assembly of Mog-V molecules, which increased the solubility of CUR by nearly 6000 times compared with pure CUR crystals. In rats, the solid dispersion increased the oral absorption of CUR by 29 folds, compared with CUR crystals. In terms of solubilisation mechanism, it was found that Mog-V self-assembled into micelles with a core-shell structure and CUR molecules were incorporated into the hydrophobic core of the Mog-V micelles.
Conclusion: Mog-V can form a solid dispersion with CUR. Upon dissolution in water, the Mog-V in the solid dispersion can self-assemble into micelles, which solubilise CUR and increase its oral absorption.
Keywords: Mogroside V; bioavailability; curcumin; micelle; solid dispersion; solubilisation.
© 2023 Zhang et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

