Mechanoenzymology in the Kinetic Resolution of β-Blockers: Propranolol as a Case Study
- PMID: 36855594
- PMCID: PMC9955203
- DOI: 10.1021/acsorginorgau.1c00049
Mechanoenzymology in the Kinetic Resolution of β-Blockers: Propranolol as a Case Study
Abstract
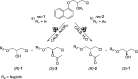
Recent advances in biotechnology, protein engineering, and enzymatic immobilization have made it possible to carry out biocatalytic transformations through alternative non-conventional activation strategies. In particular, mechanoenzymology (i.e., the use of the mechanical force produced by milling or grinding to activate a biotransformation) has become a new area in so-called "green chemistry", reshaping key fundaments of biocatalysis and leading to the exploration of enzymatic transformations under more sustainable conditions. Significantly, numerous chiral active pharmaceutical ingredients have been synthesized via mechanoenzymatic methods, boosting the use of biocatalysis in the synthesis of chiral drugs. In this regard and aiming to widen the scope of the young field of mechanoenzymology, a dual kinetic resolution of propranolol precursors was explored. The biocatalytic methodology mediated by Candida antarctica Lipase B (CALB) and activated by mechanical force allowed the isolation of both enantiomeric precursors of propranolol with high enantiomeric excess (up to 99% ee), complete conversion (c = 50%), and excellent enantiodifferentiation (E > 300). Moreover, the enantiomerically pure products were used to synthesize both enantiomers of the β-blocker propranolol with high enantiopurity.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




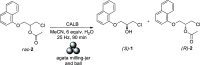
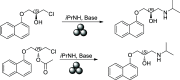
Similar articles
-
Enzymatic Kinetic Resolution of Racemic 1-(Isopropylamine)-3-phenoxy-2-propanol: A Building Block for β-Blockers.Int J Mol Sci. 2024 Oct 5;25(19):10730. doi: 10.3390/ijms251910730. Int J Mol Sci. 2024. PMID: 39409060 Free PMC article.
-
Mechanoenzymology: State of the Art and Challenges towards Highly Sustainable Biocatalysis.ChemSusChem. 2021 Jul 6;14(13):2682-2688. doi: 10.1002/cssc.202100624. Epub 2021 May 10. ChemSusChem. 2021. PMID: 33882180
-
Biocatalytic Approach for the Synthesis of Enantiopure Acebutolol as a β₁-Selective Blocker.Chirality. 2015 Jun;27(6):382-91. doi: 10.1002/chir.22444. Epub 2015 May 15. Chirality. 2015. PMID: 25977108
-
Recent Advances in Lipase-Mediated Preparation of Pharmaceuticals and Their Intermediates.Int J Mol Sci. 2015 Dec 11;16(12):29682-716. doi: 10.3390/ijms161226191. Int J Mol Sci. 2015. PMID: 26690428 Free PMC article. Review.
-
Biocatalytic approaches to optically active beta-blockers.Curr Med Chem. 2007;14(1):53-65. doi: 10.2174/092986707779313480. Curr Med Chem. 2007. PMID: 17266567 Review.
Cited by
-
Enzymatic Kinetic Resolution of Racemic 1-(Isopropylamine)-3-phenoxy-2-propanol: A Building Block for β-Blockers.Int J Mol Sci. 2024 Oct 5;25(19):10730. doi: 10.3390/ijms251910730. Int J Mol Sci. 2024. PMID: 39409060 Free PMC article.
References
-
- Erythropel H. C.; Zimmerman J. B.; de Winter T. M.; Petitjean L.; Melnikov F.; Lam C. H.; Lounsbury A. W.; Mellor K. E.; Janković N. Z.; Tu Q.; Pincus L. N.; Falinski M. M.; Shi W.; Coish P.; Plata D. L.; Anastas P. T. The Green ChemisTREE: 20 years after taking root with the 12 principles. Green Chem. 2018, 20, 1929–1961. 10.1039/c8gc00482j. - DOI
-
- Bell E. L.; Finnigan W.; France S. P.; Green A. P.; Hayes M. A.; Hepworth L. J.; Lovelock S. L.; Niikura H.; Osuna S.; Romero E.; Ryan K. S.; Turner N. J.; Flitsch S. L. Biocatalysis. Nat. Rev. Methods Primers 2021, 1, 1–46. 10.1038/s43586-021-00044-z. - DOI
-
- Gupta R. D. Recent advances in enzyme promiscuity. Sustainable Chem. Processes 2016, 4, 1–7. 10.1186/s40508-016-0046-9. - DOI
-
- Facin B. R.; Melchiors M. S.; Valério A.; Oliveira J. V.; Oliveira D. d. Driving Immobilized Lipases as Biocatalysts: 10 Years State of the Art and Future Prospects. Ind. Eng. Chem. Res. 2019, 58, 5358–5378. 10.1021/acs.iecr.9b00448. - DOI
LinkOut - more resources
Full Text Sources
