An update on COVID-19: SARS-CoV-2 variants, antiviral drugs, and vaccines
- PMID: 36855648
- PMCID: PMC9946785
- DOI: 10.1016/j.heliyon.2023.e13952
An update on COVID-19: SARS-CoV-2 variants, antiviral drugs, and vaccines
Abstract
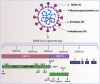
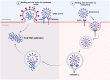
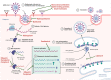
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a highly contagious and pathogenic virus that first appeared in late December 2019. This SARS-CoV-2 causes an infection of an acute respiratory disease called "coronavirus infectious disease-2019 (COVID-19). The World Health Organization (WHO) declared this SARS-CoV-2 outbreak a great pandemic on March 11, 2020. As of January 31, 2023, SARS-CoV-2 recorded more than 67 million cases and over 6 million deaths. Recently, novel mutated variants of SARS-CoV are also creating a serious health concern worldwide, and the future novel variant is still mysterious. As infection cases of SARS-CoV-2 are increasing daily, scientists are trying to combat the disease using numerous antiviral drugs and vaccines against SARS-CoV-2. To our knowledge, this is the first comprehensive review that summarized the dynamic nature of SARS-CoV-2 transmission, SARS-CoV-2 variants (a variant of concern and variant of interest), antiviral drugs and vaccines utilized against SARS-CoV-2 at a glance. Hopefully, this review will enable the researcher to gain knowledge on SARS-CoV-2 variants and vaccines, which will also pave the way to identify efficient novel vaccines against forthcoming SARS-CoV-2 strains.
Keywords: ACE2, Angiotensin-converting enzyme 2; Antiviral drugs; COVID-19; COVID-19, Coronavirus infectious disease-2019; EUA, Emergency Use Authorization; FDA, Food and Drug Administration; NIH, National Institutes of Health; RBD, Receptor-binding domain; SARS-CoV-2; SARS-CoV-2 variants; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; VOC, Variants of Concern; VOI, Variants of Interests; Vaccines; WHO, World Health Organization.
© 2023 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Organization W.H. vol. 73. 2020. (Coronavirus Disease 2019 (COVID-19): Situation Report).
-
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y., Ma X., Zhan F., Wang L., Hu T., Zhou H., Hu Z., Zhou W., Zhao L., Chen J., Meng Y., Wang J., Lin Y., Yuan J., Xie Z., Ma J., Liu W.J., Wang D., Xu W., Holmes E.C., Gao G.F., Wu G., Chen W., Shi W., Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. - DOI - PMC - PubMed
-
- Kahn J.S., McIntosh K. History and recent advances in coronavirus discovery. Pediatr. Infect. Dis. J. 2005;24:S223–S227. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

