Chemical Evaluation and Performance Characterization of Pentaerythritol Tetranitrate (PETN) under Melt Conditions
- PMID: 36855707
- PMCID: PMC9928408
- DOI: 10.1021/acsmaterialsau.2c00022
Chemical Evaluation and Performance Characterization of Pentaerythritol Tetranitrate (PETN) under Melt Conditions
Abstract
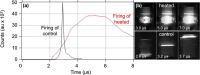
Pentaerythritol tetranitrate (PETN) is a nitrate ester explosive commonly used in commercial detonators. Although its degradation properties have been studied extensively, very little information has been collected on its thermal stability in the molten state due to the fact that its melting point is only ∼20 °C below its onset of decomposition. Furthermore, studies that have been performed on PETN thermal degradation often do not fully characterize or quantify the decomposition products. In this study, we heat PETN to melt temperatures and identify thermal decomposition products, morphology changes, and mass loss by ultrahigh-pressure liquid chromatography coupled to quadrupole time of flight mass spectrometry, scanning electron microscopy, nuclear magnetic resonance spectroscopy, and differential scanning calorimetry. For the first time, we quantify several decomposition products using independently prepared standards and establish the resulting melting point depression after the first melt. We also estimate the amount of decomposition relative to sublimation that we measure through gas evolution and evaluate the performance behavior of the molten material in commercial detonator configurations.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








References
-
- Freye C. E.; Nguyen T.-A. D.; Tappan B. C. Investigation of the Impurities in Erythritol Tetranitrate (ETN) Using UHPLC-QTOF. Prop. Explos. Pyrotech. 2021, 46, 1555–1560. 10.1002/prep.202100193. - DOI
-
- Manner V. W.; Tappan B. C.; Scott B. L.; Preston D. N.; Brown G. W. Crystal structure, packing analysis and structural-sensitivity correlations of erythritol tetranitrate. Cryst. Growth Des. 2014, 14, 6154–6160. 10.1021/cg501362b. - DOI
-
- McLennan L.; Brown-Nash A.; Busby T.; Canaria J.; Kominia A.; Smith J. L.; Oxley J. C.; Dubnikov F.; Kosloff R.; Zeiri Y. Characterization of the hexanitrate esters of sugar alcohols. Prop. Explos. Pyrotech. 2021, 46, 579–592. 10.1002/prep.202000197. - DOI
LinkOut - more resources
Full Text Sources
