Stereoselective Synthesis of Tri- and Tetrasubstituted Olefins via 1,6-Additions of Diazo Compounds and Their Precursors to p-Quinone Methides
- PMID: 36855755
- PMCID: PMC9954373
- DOI: 10.1021/acsorginorgau.1c00012
Stereoselective Synthesis of Tri- and Tetrasubstituted Olefins via 1,6-Additions of Diazo Compounds and Their Precursors to p-Quinone Methides
Abstract
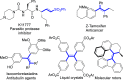
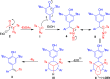
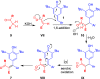
Reactions of para-quinone methides (p-QMs) with α-diazo-β-ketosulfones and their corresponding esters as well as simple β-dicarbonyl compounds and β-ketosulfones have been carried out under basic conditions. While the reaction of diazosulfone with p-QMs afforded trisubstituted olefins via deacylative 1,6-addition and elimination, α-diazo-β-ketoesters and various active methylene compounds such as 1,3-dicarbonyls and β-ketosulfones afforded tetrasubstituted olefins via 1,6-addition and aerial oxidation. These simple, environmentally benign, and mechanistically diverse protocols provided the products in moderate to excellent yields and selectivities.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








References
-
- Piva O.; Katritzky A. R.; Taylor R. J. K.. One or More C:C bond(s) by Elimination of Hydrogen, Carbon, Halogen, or Oxygen Functions. Comprehensive Organic Functional Group Transformations II; Elsevier, 2005; Vol. 1, pp 581–600.
LinkOut - more resources
Full Text Sources
