Carbon Nanotube PtSn Nanoparticles for Enhanced Complete Biocatalytic Oxidation of Ethylene Glycol in Biofuel Cells
- PMID: 36855769
- PMCID: PMC9888613
- DOI: 10.1021/acsmaterialsau.1c00029
Carbon Nanotube PtSn Nanoparticles for Enhanced Complete Biocatalytic Oxidation of Ethylene Glycol in Biofuel Cells
Abstract
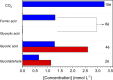
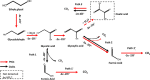
We report a hybrid catalytic system containing metallic PtSn nanoparticles deposited on multiwalled carbon nanotubes (Pt65Sn35/MWCNTs), prepared by the microwave-assisted method, coupled to the enzyme oxalate oxidase (OxOx) for complete ethylene glycol (EG) electrooxidation. Pt65Sn35/MWCNTs, without OxOx, showed good electrochemical activity toward EG oxidation and all the byproducts. Pt65Sn35/MWCNTs cleaved the glyoxilic acid C-C bond, producing CO2 and formic acid, which was further oxidized at the electrode. Concerning EG oxidation, the catalytic activity of the hybrid system (Pt65Sn35/MWCNTs+OxOx) was twice the catalytic activity of Pt65Sn35/MWCNTs. Long-term electrolysis revealed that Pt65Sn35/MWCNTs+OxOx was much more active for EG oxidation than Pt65Sn35/MWCNTs: the charge increased by 65%. The chromatographic results proved that Pt65Sn35/MWCNTs+OxOx collected all of the 10 electrons per molecule of the fuel and was able to catalyze EG oxidation to CO2 due to the associative oxidation between the metallic nanoparticles and the enzymatic pathway. Overall, Pt65Sn35/MWCNTs+OxOx proved to be a promising system to enhance the development of enzymatic biofuel cells for further application in the bioelectrochemistry field.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
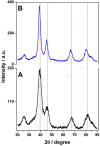
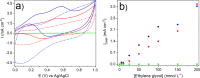
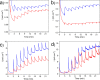
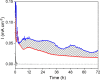

References
-
- Katz E.; Bollella P. Fuel Cells and Biofuel Cells: From Past to Perspectives. Isr. J. Chem. 2021, 61, 68–84. 10.1002/ijch.202000039. - DOI
-
- Jeerapan I.; Sempionatto J. R.; Wang J. On-Body Bioelectronics: Wearable Biofuel Cells for Bioenergy Harvesting and Self-Powered Biosensing. Adv. Funct. Mater. 2020, 30, 1906243.10.1002/adfm.201906243. - DOI
-
- Xu S.; Minteer S. D. Enzymatic Biofuel Cell for Oxidation of Glucose to CO 2. ACS Catal. 2012, 2, 91–94. 10.1021/cs200523s. - DOI
LinkOut - more resources
Full Text Sources
