Conserved allosteric inhibition mechanism in SLC1 transporters
- PMID: 36856089
- PMCID: PMC10017108
- DOI: 10.7554/eLife.83464
Conserved allosteric inhibition mechanism in SLC1 transporters
Abstract
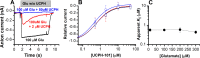
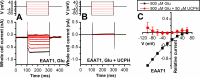
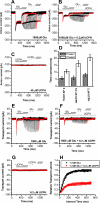
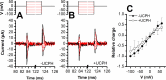
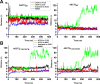
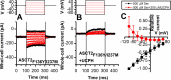
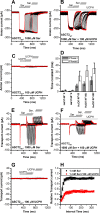
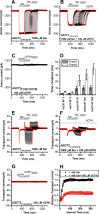
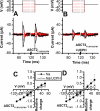
Excitatory amino acid transporter 1 (EAAT1) is a glutamate transporter belonging to the SLC1 family of solute carriers. It plays a key role in the regulation of the extracellular glutamate concentration in the mammalian brain. The structure of EAAT1 was determined in complex with UCPH-101, apotent, non-competitive inhibitor of EAAT1. Alanine serine cysteine transporter 2 (ASCT2) is a neutral amino acid transporter, which regulates pools of amino acids such as glutamine between intracellular and extracellular compartments . ASCT2 also belongs to the SLC1 family and shares 58% sequence similarity with EAAT1. However, allosteric modulation of ASCT2 via non-competitive inhibitors is unknown. Here, we explore the UCPH-101 inhibitory mechanisms of EAAT1 and ASCT2 by using rapid kinetic experiments. Our results show that UCPH-101 slows substrate translocation rather than substrate or Na+ binding, confirming a non-competitive inhibitory mechanism, but only partially inhibits wild-type ASCT2. Guided by computational modeling using ligand docking and molecular dynamics simulations, we selected two residues involved in UCPH-101/EAAT1 interaction, which were mutated in ASCT2 (F136Y, I237M, F136Y/I237M) in the corresponding positions. We show that in the F136Y/I237M double-mutant transporter, 100% of the inhibitory effect of UCPH-101 could be restored, and the apparent affinity was increased (Ki = 4.3 μM), much closer to the EAAT1 value of 0.6 μM. Finally, we identify a novel non-competitive ASCT2 inhibitor, through virtual screening and experimental testing against the allosteric site, further supporting its localization. Together, these data indicate that the mechanism of allosteric modulation is conserved between EAAT1 and ASCT2. Due to the difference in binding site residues between ASCT2 and EAAT1, these results raise the possibility that more potent, and potentially selective ASCT2 allosteric inhibitors can be designed .
Keywords: SLC1; alanine serine cysteine transporter; docking; electrophysiology; glutamate transporter; human; inhibition; molecular biophysics; rat; structural biology.
© 2023, Dong et al.
Conflict of interest statement
YD, JW, RG, KH, YS, GE, XY, AS, CG No competing interests declared
Figures








Update of
References
-
- Abrahamsen B, Schneider N, Erichsen MN, Huynh THV, Fahlke C, Bunch L, Jensen AA. Allosteric modulation of an excitatory amino acid transporter: the subtype-selective inhibitor UCPH-101 exerts sustained inhibition of EAAT1 through an intramonomeric site in the trimerization domain. The Journal of Neuroscience. 2013;33:1068–1087. doi: 10.1523/JNEUROSCI.3396-12.2013. - DOI - PMC - PubMed
-
- Arriza JL, Kavanaugh MP, Fairman WA, Wu YN, Murdoch GH, North RA, Amara SG. Cloning and expression of a human neutral amino acid transporter with structural similarity to the glutamate transporter gene family. The Journal of Biological Chemistry. 1993;268:15329–15332. doi: 10.1016/S0021-9258(18)82257-8. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

