Development of a Novel Chikungunya Virus-Like Replicon Particle for Rapid Quantification and Screening of Neutralizing Antibodies and Antivirals
- PMID: 36856407
- PMCID: PMC10101068
- DOI: 10.1128/spectrum.04854-22
Development of a Novel Chikungunya Virus-Like Replicon Particle for Rapid Quantification and Screening of Neutralizing Antibodies and Antivirals
Abstract
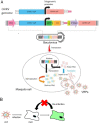
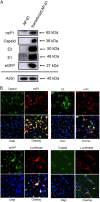
Chikungunya fever is a mosquito-transmitted infectious disease that induces rash, myalgia, and persistent incapacitating arthralgia. At present, no vaccines or antiviral therapies specific to Chikungunya virus (CHIKV) infection have been approved, and research is currently restricted to biosafety level 3 containment. CHIKV-like replicon particles (VRPs) are single-cycle infectious particles containing viral structure proteins, as well as a defective genome to provide a safe surrogate for living CHIKV to facilitate the testing of vaccines and antivirals. However, inefficient RNA transfection and the potential emergence of the competent virus through recombination in mammalian cells limit VRP usability. This study describes a transfection-free system for the safe packaging of CHIK VRP with all necessary components via transduction of mosquito cell lines using a single baculovirus vector. We observed the release of substantial quantities of mosquito cell-derived CHIK VRP (mos-CHIK VRP) from baculovirus-transduced mosquito cell lines. The VRPs were shown to recapitulate viral replication and subgenomic dual reporter expression (enhanced green fluorescent protein [eGFP] and luciferase) in infected host cells. Interestingly, the rapid expression kinetics of the VRP-expressing luciferase reporter (6 h) makes it possible to use mos-CHIK VRPs for the rapid quantification of VRP infection. Treatment with antivirals (suramin or 6-azauridine) or neutralizing antibodies (monoclonal antibodies [MAbs] or patient sera) was shown to inhibit mos-CHIK VRP infection in a dose-dependent manner. Ease of manufacture, safety, scalability, and high throughput make mos-CHIK VRPs a highly valuable vehicle for the study of CHIKV biology, the detection of neutralizing (NT) antibody activity, and the screening of antivirals against CHIKV. IMPORTANCE This study proposes a transfection-free system that enables the safe packaging of CHIK VRPs with all necessary components via baculovirus transduction. Those mosquito cell-derived CHIK VRP (mos-CHIK VRPs) were shown to recapitulate viral replication and subgenomic dual reporter (enhanced green fluorescent protein [eGFP] and luciferase) expression in infected host cells. Rapid expression kinetics of the VRP-expressing luciferase reporter (within hours) opens the door to using mos-CHIK VRPs for the rapid quantification of neutralizing antibody and antiviral activity against CHIKV. To the best of our knowledge, this is the first study to report a mosquito cell-derived alphavirus VRP system. Note that this system could also be applied to other arboviruses to model the earliest event in arboviral infection in vertebrates.
Keywords: Chikungunya virus; baculovirus; mosquito cell; virus replicon particle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Facile quantitative diagnostic testing for neutralizing antibodies against Chikungunya virus.BMC Infect Dis. 2024 Sep 30;24(1):1076. doi: 10.1186/s12879-024-09973-y. BMC Infect Dis. 2024. PMID: 39350079 Free PMC article.
-
Virus replicon particle based Chikungunya virus neutralization assay using Gaussia luciferase as readout.Virol J. 2013 Jul 15;10:235. doi: 10.1186/1743-422X-10-235. Virol J. 2013. PMID: 23855906 Free PMC article.
-
Development of a Single-Cycle Infectious SARS-CoV-2 Virus Replicon Particle System for Use in Biosafety Level 2 Laboratories.J Virol. 2022 Feb 9;96(3):e0183721. doi: 10.1128/JVI.01837-21. Epub 2021 Dec 1. J Virol. 2022. PMID: 34851142 Free PMC article.
-
Regulatory considerations in development of vaccines to prevent disease caused by Chikungunya virus.Vaccine. 2017 Sep 5;35(37):4851-4858. doi: 10.1016/j.vaccine.2017.07.065. Epub 2017 Jul 29. Vaccine. 2017. PMID: 28760614 Review.
-
Alphavirus replicon particles as candidate HIV vaccines.IUBMB Life. 2002 Apr-May;53(4-5):209-11. doi: 10.1080/15216540212657. IUBMB Life. 2002. PMID: 12120997 Review.
Cited by
-
Identification of RACK1 as a novel regulator of non-structural protein 4 of chikungunya virus.Acta Biochim Biophys Sin (Shanghai). 2024 May 29;56(10):1425-1436. doi: 10.3724/abbs.2024073. Acta Biochim Biophys Sin (Shanghai). 2024. PMID: 38813597 Free PMC article.
-
Facile quantitative diagnostic testing for neutralizing antibodies against Chikungunya virus.BMC Infect Dis. 2024 Sep 30;24(1):1076. doi: 10.1186/s12879-024-09973-y. BMC Infect Dis. 2024. PMID: 39350079 Free PMC article.
References
-
- Burt FJ, Chen W, Miner JJ, Lenschow DJ, Merits A, Schnettler E, Kohl A, Rudd PA, Taylor A, Herrero LJ, Zaid A, Ng LFP, Mahalingam S. 2017. Chikungunya virus: an update on the biology and pathogenesis of this emerging pathogen. Lancet Infect Dis 17:e107–e117. doi:10.1016/S1473-3099(16)30385-1. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

