Mitigating the detrimental developmental impact of early fetal alcohol exposure using a maternal methyl donor-enriched diet
- PMID: 36856720
- PMCID: PMC11977608
- DOI: 10.1096/fj.202201564R
Mitigating the detrimental developmental impact of early fetal alcohol exposure using a maternal methyl donor-enriched diet
Abstract
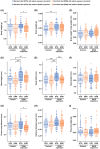
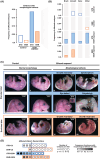
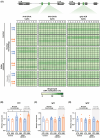
Fetal alcohol exposure at any stage of pregnancy can lead to fetal alcohol spectrum disorder (FASD), a group of life-long conditions characterized by congenital malformations, as well as cognitive, behavioral, and emotional impairments. The teratogenic effects of alcohol have long been publicized; yet fetal alcohol exposure is one of the most common preventable causes of birth defects. Currently, alcohol abstinence during pregnancy is the best and only way to prevent FASD. However, alcohol consumption remains astoundingly prevalent among pregnant women; therefore, additional measures need to be made available to help protect the developing embryo before irreparable damage is done. Maternal nutritional interventions using methyl donors have been investigated as potential preventative measures to mitigate the adverse effects of fetal alcohol exposure. Here, we show that a single acute preimplantation (E2.5; 8-cell stage) fetal alcohol exposure (2 × 2.5 g/kg ethanol with a 2h interval) in mice leads to long-term FASD-like morphological phenotypes (e.g. growth restriction, brain malformations, skeletal delays) in late-gestation embryos (E18.5) and demonstrate that supplementing the maternal diet with a combination of four methyl donor nutrients, folic acid, choline, betaine, and vitamin B12, prior to conception and throughout gestation effectively reduces the incidence and severity of alcohol-induced morphological defects without altering DNA methylation status of imprinting control regions and regulation of associated imprinted genes. This study clearly supports that preimplantation embryos are vulnerable to the teratogenic effects of alcohol, emphasizes the dangers of maternal alcohol consumption during early gestation, and provides a potential proactive maternal nutritional intervention to minimize FASD progression, reinforcing the importance of adequate preconception and prenatal nutrition.
Keywords: DNA methylation; DOHaD; FASD; embryonic development; environmental exposure; fetal alcohol exposure; maternal nutrition; methyl donors.
© 2023 The Authors. The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Figures


References
-
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;302(7836):999‐1001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

