Myeloma bone disease: pathogenesis and management in the era of new anti-myeloma agents
- PMID: 36856824
- PMCID: PMC9975874
- DOI: 10.1007/s00774-023-01403-4
Myeloma bone disease: pathogenesis and management in the era of new anti-myeloma agents
Abstract
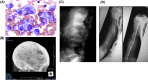
Introduction: Multiple myeloma (MM) is a malignancy of plasma cells with characteristic bone disease. Despite recent great strides achieved in MM treatment owing to the implementation of new anti-MM agents, MM is still incurable and bone destruction remains a serious unmet issue in patients with MM.
Approach: In this review, we will summarize and discuss the mechanisms of the formation of bone disease in MM and the available preclinical and clinical evidence on the treatment for MM bone disease.
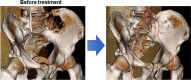
Conclusions: MM cells produce a variety of cytokines to stimulate receptor activator of nuclear factor-κB ligand-mediated osteoclastogenesis and suppress osteoblastic differentiation from bone marrow stromal cells, leading to extensive bone destruction with rapid loss of bone. MM cells alter the microenvironment through bone destruction where they colonize, which in turn favors tumor growth and survival, thereby forming a vicious cycle between tumor progression and bone destruction. Denosumab or zoledronic acid is currently recommended to be administered at the start of treatment in newly diagnosed patients with MM with bone disease. Proteasome inhibitors and the anti-CD38 monoclonal antibody daratumumab have been demonstrated to exert bone-modifying activity in responders. Besides their anti-tumor activity, the effects of new anti-MM agents on bone metabolism should be more precisely analyzed in patients with MM. Because prognosis in patients with MM has been significantly improved owing to the implementation of new agents, the therapeutic impact of bone-modifying agents should be re-estimated in the era of these new agents.
Keywords: Bone-modifying agents; Daratumumab; Myeloma bone disease; Proteasome inhibitors; Receptor activator of nuclear factor-κB ligand.
© 2023. The Japanese Society Bone and Mineral Research.
Conflict of interest statement
M.A. received research funding from Chugai Pharmaceutical, GlaxoSmithKline, Sanofi K.K., Kyowa Kirin, Nippon Shinyaku, Teijin Pharma, and Ono Pharmaceutical, and honoraria from Daiichi Sankyo, Janssen Pharmaceutical K.K., Takeda Pharmaceutical, Sanofi K.K., Bristol-Myers Squibb, and Ono Pharmaceutical. Other authors have no competing financial interests to declare.
Figures




References
-
- Pecherstorfer M, Seibel MJ, Woitge HW, Horn E, Schuster J, Neuda J, Sagaster P, Köhn H, Bayer P, Thiébaud D, Ludwig H. Bone resorption in multiple myeloma and in monoclonal gammopathy of undetermined significance: quantification by urinary pyridinium cross-links of collagen (in eng) Blood. 1997;90:3743–3750. doi: 10.1182/blood.V90.9.3743. - DOI - PubMed
-
- Hashimoto T, Abe M, Oshima T, Shibata H, Ozaki S, Inoue D, Matsumoto T. Ability of myeloma cells to secrete macrophage inflammatory protein (MIP)-1alpha and MIP-1beta correlates with lytic bone lesions in patients with multiple myeloma (in eng) Br J Haematol. 2004;125:38–41. doi: 10.1111/j.1365-2141.2004.04864.x. - DOI - PubMed
-
- Pearse RN, Sordillo EM, Yaccoby S, Wong BR, Liau DF, Colman N, Michaeli J, Epstein J, Choi Y. Multiple myeloma disrupts the TRANCE/ osteoprotegerin cytokine axis to trigger bone destruction and promote tumor progression (in eng) Proc Natl Acad Sci USA. 2001;98:11581–11586. doi: 10.1073/pnas.201394498. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

