Dry Eye Disease Associated with Meibomian Gland Dysfunction: Focus on Tear Film Characteristics and the Therapeutic Landscape
- PMID: 36856980
- PMCID: PMC10164226
- DOI: 10.1007/s40123-023-00669-1
Dry Eye Disease Associated with Meibomian Gland Dysfunction: Focus on Tear Film Characteristics and the Therapeutic Landscape
Abstract
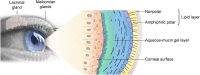
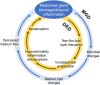
Meibomian gland dysfunction (MGD) is highly prevalent and is the leading cause of evaporative dry eye disease (DED). MGD is characterized by a reduction in meibum secretion and/or a change in meibum composition that results in the disruption of the tear film lipid layer and an increase in the tear film evaporation rate. Excessive evaporation causes tear film instability, desiccation, tear hyperosmolarity, inflammation, and apoptosis of ocular surface cells, resulting in a continuous cycle of DED. The primary treatment goal for DED associated with MGD is to restore the tear film lipid layer and decrease evaporation, thereby reducing ocular signs and symptoms. The management of MGD includes home care options (eyelid hygiene, warming eye masks, ocular lubricants) and office-based treatments (manual expression, microblepharoexfoliation, thermal pulsation, intense pulsed light, intraductal probing). Topical ophthalmic prescription medications attempt to alter various factors that may contribute to DED (e.g., inflammation, bacterial growth, inadequate tear production). In this review, clinical evidence regarding available treatments and emerging therapies from randomized studies in patients with DED associated with MGD is summarized. Although some treatment modalities have been evaluated specifically for DED patients with MGD, large-scale randomized controlled trials are needed to confirm efficacy and safety in this patient population. Currently, there are no approved prescription pharmacologic treatments specifically indicated for DED associated with MGD, and those medications approved for the treatment of DED do not target the key driver of the disease (i.e., excessive evaporation). NOV03 (perfluorohexyloctane; under review with the US Food and Drug Administration) is the most advanced emerging therapy for DED associated with MGD and has demonstrated statistically significant improvements in both signs and symptoms in randomized controlled trials. Development of novel pharmacotherapies will improve therapeutic options and allow for a more individualized approach for patients with DED associated with MGD.
Keywords: Clinical trials; Dry eye disease; Evaporation; Lipid layer; Meibomian gland dysfunction; NOV03; Perfluorohexyloctane; Pharmacotherapies; Tear film.
© 2023. The Author(s).
Conflict of interest statement
John D. Sheppard reports serving on advisory boards, speakers bureaus, or as a consultant for 1-800-DOCTORS, AbbVie, Alcon, Aldeyra Therapeutics, Aerie Pharmaceuticals, Allergan, ArcScan, Avedro, Bausch + Lomb, Bio-Tissue/TissueTech, BioLayer, Bruder Healthcare, Claris Bio, Clearside, Clearview, Clementia Pharmaceuticals, Dompé, Eleven, Eyedetec Medical, EyeGate Research, Eyevance, Glaukos, Hovione, Imprimis Pharma, Inspire/Merck Pharmaceuticals, Isis Pharmaceuticals, Johnson & Johnson/TearScience/Vistakon, Kala Pharmaceuticals, Kowa, LacriSciences Vision, LayerBio, Lenstatin, Lumenis, Lux Biosciences, Mallinckrodt, Mati Therapeutics, Mededicus, Mitotech, Nicox, NovaBay Pharmaceuticals, Novaliq, Novartis, Noveome Biotherapeutics/Stemnion, OcuCure Therapeutics, Inc., Ocular Therapeutix, Oculis, Okogen, Omeros, Oyster Point Pharma, Inc., Parion Sciences, PentaVision, Pfizer, Portage, Quidel Corporation, Rapid Pathogen Screening, Inc., Santen, ScienceBased Health, Shire, Sun Pharmaceuticals, Surrozen, Synedgen, Takeda, Talia Technology, TearLab, Topcon Healthcare, and TopiVert; conducting clinical research for AbbVie, Alcon, Aldeyra Therapeutics, Allergan, ArcScan, Avedro, Bausch + Lomb, Clearside, Clearview, Clementia Pharmaceuticals, Dompé, EyeGate Research, EyeRx Research, Fortress Biotech, Glaukos, Hovione, InSite Vision, Inc., Inspire/Merck Pharmaceuticals, Isis Pharmaceuticals, Johnson & Johnson/TearScience/Vistakon, Kala Pharmaceuticals, LacriSciences Vision, Lux Biosciences, Mimetogen, Mitotech, NeoMedix, Novaliq, Novartis, Ocular Therapeutix, Okogen, Oyster Point Pharma, Inc., Parion Sciences, Pfizer, Rapid Pathogen Screening Inc., Rutech, Santen, Senju Pharmaceutical Co., Shire, TearSolutions, Inc., Topcon Healthcare, and Xoma/Servier; investor, shareholder, or stock interests in 1-800-DOCTORS, Alphaeon (Parent company: Strathspey Crowne), BioLayer, Eyedetec Medical, EyeGate Research, EyeRx Research, Eyevance, LacriSciences Vision, LayerBio, Mati Therapeutics, NovaBay Pharmaceuticals, Noveome Biotherapeutics/Stemnion, OccuHub, OcuCure, Inc., Okogen, Oyster Point Pharma, Inc., Rapid Pathogen Screening, Inc., Shire, and TearLab. Kelly K. Nichols reports receiving research funding from Aramis Biosciences, Kowa, ScienceBased Health, Sylentis, and TearScience; and serving as a consultant to and receiving honoraria from Allergan/AbbVie, Aerie Pharmaceuticals, Aldeyra Therapeutics, Bausch + Lomb, Bruder, Dompé, HanAll Biopharma, Iveric Bio, Kala Pharmaceuticals, Novartis/Shire, Osmotica/RVL Pharmaceuticals, Oyster Point Pharma, Inc., Palatin Technologies, Sight Sciences, Inc., Tarsus, Tear Film Innovations/Alcon/Acquiom, TearSolutions, Inc., Thea, Verséa Ophthalmics, Visionology, Xequel Bio, and Yuyu Pharma.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

