Effect of an Exclusive Human Milk Diet on the Gut Microbiome in Preterm Infants: A Randomized Clinical Trial
- PMID: 36857051
- PMCID: PMC9978942
- DOI: 10.1001/jamanetworkopen.2023.1165
Effect of an Exclusive Human Milk Diet on the Gut Microbiome in Preterm Infants: A Randomized Clinical Trial
Abstract
Importance: The effect of using an exclusive human milk diet compared with one that uses bovine products in preterm infants is uncertain, but some studies demonstrate lower rates of key neonatal morbidities. A potential mediating pathway is the gut microbiome.
Objective: To determine the effect of an exclusive human milk diet on gut bacterial richness, diversity, and proportions of specific taxa in preterm infants from enrollment to 34 weeks' postmenstrual age.
Design, setting, and participants: In this randomized clinical trial conducted at 4 neonatal intensive care units in the United Kingdom from 2017 to 2020, microbiome analyses were blind to group. Infants less than 30 weeks' gestation who had only received own mother's milk were recruited before 72 hours of age. Statistical analysis was performed from July 2019 to September 2021.
Interventions: Exclusive human milk diet using pasteurized human milk for any shortfall in mother's own milk supply and human milk-derived fortifiers (intervention) compared with bovine formula and bovine-derived fortifier (control) until 34 weeks' postmenstrual age. Fortifier commenced less than 48 hours of tolerating 150 mL/kg per day.
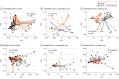
Main outcomes and measures: Gut microbiome profile including alpha and beta diversity, and presence of specific bacterial taxa.
Results: Of 126 preterm infants enrolled in the study, 63 were randomized to control (median [IQR] gestation: 27.0 weeks [26.0-28.1 weeks]; median [IQR] birthweight: 910 g [704-1054 g]; 32 [51%] male) and 63 were randomized to intervention (median [IQR] gestation: 27.1 weeks [25.7-28.1 weeks]; median [IQR] birthweight: 930 g [733-1095 g]; 38 [60%] male); 472 stool samples from 116 infants were analyzed. There were no differences in bacterial richness or Shannon diversity over time, or at 34 weeks between trial groups. The exclusive human milk diet group had reduced relative abundance of Lactobacillus after adjustment for confounders (coefficient estimate, 0.056; P = .03), but not after false discovery rate adjustment. There were no differences in time to full feeds, necrotizing enterocolitis, or other key neonatal morbidities.
Conclusions and relevance: In this randomized clinical trial in preterm infants using human milk-derived formula and/or fortifier to enable an exclusive human milk diet, there were no effects on overall measures of gut bacterial diversity but there were effects on specific bacterial taxa previously associated with human milk receipt. These findings suggest that the clinical impact of human milk-derived products is not modulated via microbiomic mechanisms.
Trial registration: ISRCTN trial registry identifier: ISRCTN16799022.
Conflict of interest statement
Figures