Successful treatment of non-Langerhans cell histiocytosis with the MEK inhibitor trametinib: a multicenter analysis
- PMID: 36857436
- PMCID: PMC10410131
- DOI: 10.1182/bloodadvances.2022009013
Successful treatment of non-Langerhans cell histiocytosis with the MEK inhibitor trametinib: a multicenter analysis
Abstract
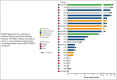
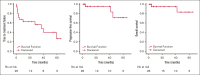
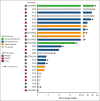
Erdheim-Chester disease (ECD) and Rosai-Dorfman disease (RDD) are rare non-Langerhans cell histiocytoses (non-LCHs), for which therapeutic options are limited. MAPK pathway activation through BRAFV600E mutation or other genomic alterations is a histiocytosis hallmark and correlates with a favorable response to BRAF inhibitors and the MEK inhibitor cobimetinib. However, there has been no systematic evaluation of alternative MEK inhibitors. To assess the efficacy and safety of the MEK inhibitor trametinib, we retrospectively analyzed the outcomes of 26 adult patients (17 with ECD, 5 with ECD/RDD, 3 with RDD, and 1 with ECD/LCH) treated with orally administered trametinib at 4 major US care centers. The most common treatment-related toxicity was rash (27% of patients). In most patients, the disease was effectively managed at low doses (0.5-1.0 mg trametinib daily). The response rate of the 17 evaluable patients was 71% (73% [8/11] without a detectable BRAFV600E achieving response). At a median follow-up of 23 months, treatment effects were durable, with a median time-to-treatment failure of 37 months, whereas the median progression-free and overall survival were not reached (at 3 years, 90.1% of patients were alive). Most patients harbored mutations in BRAF (either classic BRAFV600E or other BRAF alterations) or alterations in other genes involved in the MAPK pathway, eg, MAP2K, NF1, GNAS, or RAS. Most patients required lower than standard doses of trametinib but were responsive to lower doses. Our data suggest that the MEK inhibitor trametinib is an effective treatment for ECD and RDD, including those without the BRAFV600E mutation.
© 2023 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: E.L.D. receives unpaid editorial support from Pfizer Inc. and paid advisory board membership with Day One Biopharmaceuticals, Springworks Therapeutics, and Opna Bio, all outside the submitted work. R.K. has received research funding from Biological Dynamics, Boehringer Ingelheim, Debiopharm, Foundation Medicine, Genentech, Grifols, Guardant Health, Incyte Corporation, Konica Minolta, Medimmune, Merck Serono, OmniSeq, Pfizer, Sequenom, Takeda, and TopAlliance; consultantcy, speaker fees, and/or advisory board for Actuate Therapeutics, AstraZeneca, Bicara Therapeutics, Biological Dynamics, Caris, Datar Cancer Genetics, Eisai, EOM Pharmaceuticals, Iylon, Merck, NeoGenomics, Neomed, Pfizer, Prosperdtx, Roche, TD2/Volastra, Turning Point Therapeutics, and XBiotech; has an equity interest in CureMatch, CureMetrix, and IDbyDNA; serves on the board of CureMatch and CureMetrix; and is a cofounder of CureMatch. F.J. reports research support from Astex, Novartis, BioMed Valley Discoveries, Fore Biotherapeutics, Deciphera, Bristol Myers Squibb, Asana, IDEAYA Biosciences, Sanofi, Merck, F-star, JS InnoPharm, BioXcel, Lilly, Bicara Therapeutics, PureTech Health, FUJIFILM Pharmaceuticals, SOTIO, Synlogic, NextCure, and Hutchinson MediPharma; is on the scientific advisory boards of IDEAYA Biosciences, Synlogic, SOTIO, PureTech Health, Deciphera, Crown Bioscience, Asana, Fore Biotherapeutics, Novartis, Bicara Therapeutics, and PegaOne; is a paid consultant for Mersana Therapeutics, Flame Biosciences, Cardiff Oncology, MedinCell, and ImmunoMet Therapeutics; and has an ownership interest in Cardiff Oncology. The remaining authors declare no competing financial interests.
Figures
References
-
- Munoz J, Janku F, Cohen PR, Kurzrock R. Erdheim-Chester disease: characteristics and management. Mayo Clin Proc. 2014;89(7):985–996. - PubMed
-
- Haroche J, Amoura Z, Wechsler B, Veyssier-Belot C, Charlotte F, Piette JC. [Erdheim-Chester disease] La Presse Medicale. 2007;36(11):1663–1668. - PubMed
-
- Andre M, Delevaux I, de Fraissinette B, et al. Two enlarged kidneys: a manifestation of Erdheim-Chester disease. Am J Nephrol. 2001;21(4):315–317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous