Functional imaging and quantification of multineuronal olfactory responses in C. elegans
- PMID: 36857454
- PMCID: PMC9977185
- DOI: 10.1126/sciadv.ade1249
Functional imaging and quantification of multineuronal olfactory responses in C. elegans
Abstract
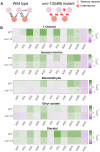
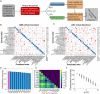
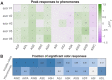
Many animals perceive odorant molecules by collecting information from ensembles of olfactory neurons, where each neuron uses receptors that are tuned to recognize certain odorant molecules with different binding affinity. Olfactory systems are able, in principle, to detect and discriminate diverse odorants using combinatorial coding strategies. We have combined microfluidics and multineuronal imaging to study the ensemble-level olfactory representations at the sensory periphery of the nematode Caenorhabditis elegans. The collective activity of C. elegans chemosensory neurons reveals high-dimensional representations of olfactory information across a broad space of odorant molecules. We reveal diverse tuning properties and dose-response curves across chemosensory neurons and across odorants. We describe the unique contribution of each sensory neuron to an ensemble-level code for volatile odorants. We show that a natural stimuli, a set of nematode pheromones, are also encoded by the sensory ensemble. The integrated activity of the C. elegans chemosensory neurons contains sufficient information to robustly encode the intensity and identity of diverse chemical stimuli.
Figures




References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

