Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary
- PMID: 36858443
- PMCID: PMC10066569
- DOI: 10.1183/13993003.00239-2023
Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary
Abstract
Executive summary of the Global Strategy for Prevention, Diagnosis and Management of COPD 2023: the latest evidence-based strategy document from the Global Initiative for Chronic Obstructive Lung Disease (GOLD) https://bit.ly/3KCaTGe
Conflict of interest statement
Conflict of interest: A. Agustí is Chair of the Board of Directors of GOLD (no payment received), and reports grants or contracts from AZ, GSK, Chiesi and Menarini, consultancy fees from AZ, GSK, Chiesi, Menarini, Zambon, MSD and Sanofi, and payment or honoraria for lectures, presentations, manuscript writing or educational events from AZ, GSK, Chiesi, Menarini and Zambon, outside the submitted work. B.R. Celli reports support for the present work from Chiesi Farmaceutici; grants or contracts from GlaxoSmithKline, AstraZeneca, Menarini, Sanofi Aventis and Axios, consultancy fees from GlaxoSmithKline, AstraZeneca and Sanofi Aventis, payment or honoraria for lectures, presentations, manuscript writing or educational events from GlaxoSmithKline, AstraZeneca, Menarini, Chiesi and Regeneron, support for attending meetings and/or travel from GlaxoSmithKline and Sanofi Aventis, and participation on a data safety monitoring board or advisory board for AZ Therapeutics, Sanofi Aventis and Vertex, outside the submitted work. G.J. Criner reports support for the present work from GlaxoSmithKline; grants or contracts from ALung Technologies Inc., American College of Radiology, American Lung Association, AstraZeneca, BioScale Inc., Boehringer Ingelheim, BREATH Therapeutics Inc., COPD Foundation, Coridea/ZIDAN, Corvus, Dr Karen Burns of St Michael's Hospital, Fisher & Paykel Healthcare Ltd, Galapagos NV, GlaxoSmithKline, Kinevent, Lungpacer Medical Inc., National Heart, Lung, and Blood Institute, Nurvaira Inc., Patient-Centered Outcomes Research Institute, Pulmonary Fibrosis Foundation, PulmonX, Respironics Inc., Respivant Sciences, Spiration Inc., Steward St Elizabeth's Medical Center of Boston Inc. and Veracyte Inc., and personal fees from Amgen, AstraZeneca, Boehringer Ingelheim, Broncus Medical, CSA Medical, EOLO Medical, Gala Therapeutics, GlaxoSmithKline, Helios Medical, Ion, Merck, Medtronic, Mereo BioPharma, NGM Biopharmaceuticals, Novartis, Olympus, PulmonX, Respironics Inc., Respivant Sciences, The Implementation Group and Verona Pharma, outside the submitted work. D. Halpin reports payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Novartis, Pfizer, Sanofi and Menarini, support for attending meetings and/or travel from Menarini, and participation on a data safety monitoring board or advisory board with Chiesi, outside the submitted work. A. Anzueto reports consultancy fees from GlaxoSmithKline, AstraZeneca and Boehringer Ingelheim, and payment or honoraria for lectures, presentations, manuscript writing or educational events from Viatrix Pharma, outside the submitted work. P. Barnes reports grants or contracts from AstraZeneca and Boehringer Ingelheim, consultancy fees from AstraZeneca, Boehringer Ingelheim, Novartis, Teva and Epi-Endo, and payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, Boehringer Ingelheim, Novartis and Teva, outside the submitted work. J. Bourbeau reports grants or contracts from Canadian Institute of Heath Research (CIHR), Réseau en santé respiratoire du FRQS, McGill University, McGill University Health Centre Foundation, AstraZeneca Canada Ltd, Boehringer Ingelheim Canada Ltd, GlaxoSmithKline Canada Ltd, Grifols, Novartis, Sanofi and Trudell Canada Ltd, and payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca Canada Ltd, COVIS Pharma Canada Ltd, GlaxoSmithKline Canada Ltd, Pfizer Canada Ltd and Trudell Canada Ltd, outside the submitted work. M.K. Han reports grants or contracts from NIH, Sanofi, Novartis, Nuvaira, Sunovion, Gala Therapeutics, COPD Foundation, AstraZeneca, American Lung Association, Boehringer Ingelheim and Biodesix, royalties or licences from Uptodate, Norton Publishing and Penguin Random House, consultancy fees from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Pulmonx, Teva, Verona, Merck, Mylan, Sanofi, DevPro, Aerogen, Polarian, United Therapeutics, Regeneron, Altesa BioPharma and Amgen, payment or honoraria for lectures, presentations, manuscript writing or educational events from Cipla, Chiesi, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Medscape, Integrity and NACE, participation on a data safety monitoring board or advisory board with Novartis and Medtronic, leadership or fiduciary roles with COPD Foundation, COPD Foundation Scientific Advisory Committee, ALA advisory committee, American Thoracic Society (journal editor), ALA (volunteer spokesperson), GOLD scientific committee and Emerson School Board, stock or stock options with Meissa Vaccines and Altesa BioPharma, receipt of equipment, materials, drugs, medical writing, gifts or other services from GSK, Boehringer Ingelheim, AstraZeneca and Novartis, and personal fees from Medscape and Integrity, outside the submitted work. F.J. Martinez reports grants or contracts from AstraZeneca, Chiesi, GSK and Sanofi/Regeneron, consultancy fees from AstraZeneca, Boehringer Ingelheim, Chiesi, CSL Behring, GSK, Novartis, Polarean, Pulmonx, Sanofi/Regeneron, Sunovion, Teva, Theravance/Viatris and UpToDate, payment or honoraria for lectures, presentations, manuscript writing or educational events from GSK and AstraZeneca, and participation on a data safety monitoring board or advisory board for MedTronic and GSK, outside the submitted work. M. Montes de Oca reports payment or honoraria for lectures, presentations, manuscript writing or educational events from GlaxoSmithKline and AstraZeneca, outside the submitted work. K. Mortimer has contributed to advisory boards for AstraZeneca and GlaxoSmithKline, outside the submitted work. A. Papi reports grants or contracts from Chiesi, AstraZeneca, GSK, Sanofi and Agenzia Italiana del Farmaco (AIFA), consultancy fees from Chiesi, AstraZeneca, GSK, Novartis, Sanofi, Avillion and Elpen Pharmaceuticals, payment or honoraria for lectures, presentations, manuscript writing or educational events from Chiesi, AstraZeneca, GSK, Menarini, Novartis, Zambon, Mundipharma, Sanofi, Edmond Pharma, Iqvia, Avillion and Elpen Pharmaceuticals, and participation on a data safety monitoring board or advisory board for Chiesi, AstraZeneca, GSK, MSD, Novartis, Sanofi, Iqvia, Avillion and Elpen Pharmaceuticals, outside the submitted work. I. Pavord reports speaker fees from Aerocrine AB, speaker and consultancy fees from Almirall and Novartis, speaker fees, payments for organization of educational events, consultancy fees and international scientific meeting sponsorship from AstraZeneca, GSK, Regeneron Pharmaceuticals, Inc., Sanofi and Teva, speaker fees, consultancy fees and international scientific meeting sponsorship from Boehringer Ingelheim, speaker fees, consultancy fees, research grants and international scientific meeting sponsorship from Chiesi, consultancy fees from Circassia, Dey Pharma, Genentech, Knopp Biosciences, Merck, MSD, RespiVert and Schering-Plough, and consultancy fees and international scientific meeting sponsorship from Napp Pharmaceuticals, outside the submitted work. N. Roche reports grants or contracts from Boehringer Ingelheim, Novartis, GSK and Pfizer, consultancy fees from Boehringer Ingelheim, GSK, AstraZeneca, Sanofi, Chiesi, Pfizer, Novartis, Teva and Bayer, and payment or honoraria for lectures, presentations, manuscript writing or educational events from Boehringer Ingelheim, GSK, AstraZeneca, Sanofi, Chiesi, Pfizer, Novartis, Teva, Zambon and MSD, outside the submitted work. D.D. Sin reports payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, Boehringer Ingelheim and GSK, outside the submitted work. D. Singh reports consultancy fees from Aerogen, AstraZeneca, Boehringer Ingelheim, Chiesi, Cipla, CSL Behring, Epiendo, Genentech, GlaxoSmithKline, Glenmark, Gossamerbio, Kinaset, Menarini, Novartis, Pulmatrix, Sanofi, Synairgen, Teva, Theravance and Verona, outside the submitted work. R. Stockley reports consultancy fees from CSL Behring and Mereo Biopharma, and participation on a data safety monitoring board or advisory board for Kamada and Syneos, outside the submitted work. J.A. Wedzicha reports grants or contracts from AstraZeneca, Boehringer, Chiesi, GSK, Novartis, Genentech and 37Clinical, consultancy fees from AstraZeneca, Epiendo, GSK, Gilead, Novartis, Pieris and Pulmatrix, payment or honoraria for lectures, presentations, manuscript writing or educational events from AstraZeneca, GSK, Boehringer, Recipharm and Novartis, and participation on a data safety monitoring board or advisory board for Virtus, outside the submitted work. C.F. Vogelmeier reports grants or contracts from German Ministry of Education and Science (BMBF), AstraZeneca, Boehringer Ingelheim, Chiesi, CSL Behring, GlaxoSmithKline, Grifols and Novartis, consultancy fees from Aerogen, AstraZeneca, Boehringer Ingelheim, CSL Behring, Chiesi, GlaxoSmithKline, Insmed, Menarini, Novartis and Nuvaira, and payment or honoraria for lectures, presentations, manuscript writing or educational events from Aerogen, AstraZeneca, Boehringer Ingelheim, CSL Behring, Chiesi, GlaxoSmithKline, Insmed, Menarini, Novartis, Roche and Sanofi, outside the submitted work. The remaining authors have no potential conflicts of interest to disclose.
Figures
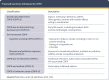
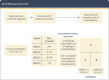
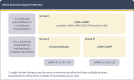
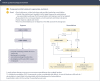


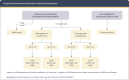
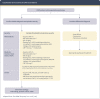
Comment in
-
Pushing (for) GOLD.Eur Respir J. 2023 Apr 1;61(4):2300366. doi: 10.1183/13993003.00366-2023. Print 2023 Apr. Eur Respir J. 2023. PMID: 37003615 No abstract available.
-
The GOLD 2023 proposed taxonomy: a new tool to determine COPD etiotypes.Eur Respir J. 2023 Jun 8;61(6):2300466. doi: 10.1183/13993003.00466-2023. Print 2023 Jun. Eur Respir J. 2023. PMID: 37290808 No abstract available.
-
Implication of the Global Initiative for Chronic Obstructive Lung Disease 2023 report for resource-limited settings: tracing the G in the GOLD.Eur Respir J. 2023 Jun 15;61(6):2300484. doi: 10.1183/13993003.00484-2023. Print 2023 Jun. Eur Respir J. 2023. PMID: 37321612 Free PMC article.
-
GOLD 2023 Executive Summary: responses from the GOLD Scientific Committee.Eur Respir J. 2023 Jun 15;61(6):2300616. doi: 10.1183/13993003.00616-2023. Print 2023 Jun. Eur Respir J. 2023. PMID: 37321613 Free PMC article.
-
Questioning the purpose of annual follow-up spirometry for all patients with COPD.Eur Respir J. 2023 Jun 15;61(6):2300292. doi: 10.1183/13993003.00292-2023. Print 2023 Jun. Eur Respir J. 2023. PMID: 37321614 No abstract available.
-
Shorter duration of antibiotic therapy for exacerbation of COPD.Eur Respir J. 2023 Jun 15;61(6):2300455. doi: 10.1183/13993003.00455-2023. Print 2023 Jun. Eur Respir J. 2023. PMID: 37321615 No abstract available.
-
Can we call all obstructive lung diseases COPD?Eur Respir J. 2023 Jun 15;61(6):2300462. doi: 10.1183/13993003.00462-2023. Print 2023 Jun. Eur Respir J. 2023. PMID: 37321616 No abstract available.
-
GOLD 2023 executive summary: comments from Asia's perspective.Eur Respir J. 2023 Jun 15;61(6):2300562. doi: 10.1183/13993003.00562-2023. Print 2023 Jun. Eur Respir J. 2023. PMID: 37321617 No abstract available.
References
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for Prevention, Diagnosis and Management of COPD: 2023 Report. https://goldcopd.org/2023-gold-report-2/
-
- Global Initiative for Chronic Obstructive Lung Disease. 2022 Global Strategy for Prevention, Diagnosis and Management of COPD. www.goldcopd.org
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
