TFAM deficiency in dendritic cells leads to mitochondrial dysfunction and enhanced antitumor immunity through cGAS-STING pathway
- PMID: 36858460
- PMCID: PMC9980377
- DOI: 10.1136/jitc-2022-005430
TFAM deficiency in dendritic cells leads to mitochondrial dysfunction and enhanced antitumor immunity through cGAS-STING pathway
Abstract
Background: Mitochondrial transcription factor A (TFAM) is a transcription factor that maintains mitochondrial DNA (mtDNA) stabilization and initiates mtDNA replication. However, little is known about the immune regulation function and TFAM expression in immune cells in the tumors.
Methods: Mouse tumor models were applied to analyze the effect of TFAM deficiency in myeloid cell lineage on tumor progression and tumor microenvironment (TME) modification. In vitro, primary mouse bone marrow-derived dendritic cells (BMDCs) were used in the investigation of the altered function and the activated pathway. OVA was used as the model antigen to validate the activation of immune responses in vivo. STING inhibitors were used to confirm the STING activation provoked by Tfam deficient in DCs.
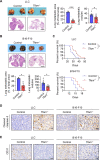
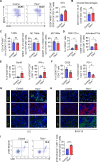
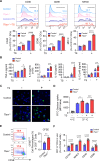
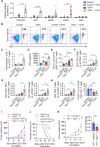
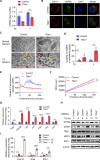
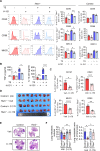
Results: The deletion of TFAM in DCs led to mitochondrial dysfunction and mtDNA cytosolic leakage resulting in the cGAS-STING pathway activation in DCs, which contributed to the enhanced antigen presentation. The deletion of TFAM in DCs has interestingly reversed the immune suppressive TME and inhibited tumor growth and metastasis in tumor models.
Conclusions: We have revealed that TFAM knockout in DCs ameliorated immune-suppressive microenvironment in tumors through STING pathway. Our work suggests that specific TFAM knockout in DCs might be a compelling strategy for designing novel immunotherapy methods in the future.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: No, there are no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
