Sirtuin3 confers protection against acute pulmonary embolism through anti-inflammation, and anti-oxidative stress, and anti-apoptosis properties: participation of the AMP-activated protein kinase/mammalian target of rapamycin pathway
- PMID: 36858596
- PMCID: PMC10435360
- DOI: 10.1538/expanim.22-0175
Sirtuin3 confers protection against acute pulmonary embolism through anti-inflammation, and anti-oxidative stress, and anti-apoptosis properties: participation of the AMP-activated protein kinase/mammalian target of rapamycin pathway
Abstract
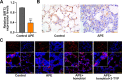
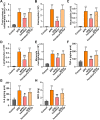
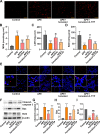
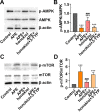
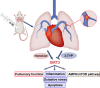
An increasing number of studies have suggested that oxidative stress and inflammation play momentous roles in acute pulmonary embolism (APE). Honokiol, a bioactive biphenolic phytochemical substance, is known for its strong anti-oxidative and anti-inflammatory effects, and it served as an activator of sirtuin3 (SIRT3) in the present study. The purposes of the study were to explore the effects of honokiol on APE rats and investigate whether the function of honokiol is mediated by SIRT3 activation. In the study, the rats received a right femoral vein injection of dextran gel G-50 particles (12 mg/kg) to establish the APE model and were subsequently administered honokiol and/or a selective SIRT3 inhibitor 3-(1H-1,2,3-triazol-4-yl)pyridine (3-TYP; 5 mg/kg) intraperitoneally. The results showed that SIRT3 activation by honokiol attenuated the loss in lung function, ameliorated the inflammatory response and oxidative damage, and inhibited apoptosis in lung tissues of the rats with APE but that this was reversed by 3-TYP. In addition, we found that the AMP-activated protein kinase (AMPK)/mammalian target of rapamycin (mTOR) pathway might be activated by honokiol but restrained by 3-TYP. These results indicated that honokiol was capable of suppressing the adverse effects of APE and that this was diminished by SIRT3 suppression, implying that activation of SIRT3 might serve as a therapeutic method for APE.
Keywords: acute pulmonary embolism (APE); apoptosis; inflammation; oxidative stress; sirtuin3 (SIRT3).
Figures

References
-
- Eagleton MJ, Henke PK, Luke CE, Hawley AE, Bedi A, Knipp BS, et al. Southern Association for Vascular Surgery William J. von Leibig Award. Inflammation and intimal hyperplasia associated with experimental pulmonary embolism. J Vasc Surg. 2002; 36: 581–588. doi: 10.1067/mva.2002.126556 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

