Examining craniofacial variation among crispant and mutant zebrafish models of human skeletal diseases
- PMID: 36858797
- PMCID: PMC10273351
- DOI: 10.1111/joa.13847
Examining craniofacial variation among crispant and mutant zebrafish models of human skeletal diseases
Abstract
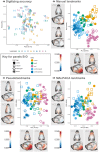
Genetic diseases affecting the skeletal system present with a wide range of symptoms that make diagnosis and treatment difficult. Genome-wide association and sequencing studies have identified genes linked to human skeletal diseases. Gene editing of zebrafish models allows researchers to further examine the link between genotype and phenotype, with the long-term goal of improving diagnosis and treatment. While current automated tools enable rapid and in-depth phenotyping of the axial skeleton, characterizing the effects of mutations on the craniofacial skeleton has been more challenging. The objective of this study was to evaluate a semi-automated screening tool can be used to quantify craniofacial variations in zebrafish models using four genes that have been associated with human skeletal diseases (meox1, plod2, sost, and wnt16) as test cases. We used traditional landmarks to ground truth our dataset and pseudolandmarks to quantify variation across the 3D cranial skeleton between the groups (somatic crispant, germline mutant, and control fish). The proposed pipeline identified variation between the crispant or mutant fish and control fish for four genes. Variation in phenotypes parallel human craniofacial symptoms for two of the four genes tested. This study demonstrates the potential as well as the limitations of our pipeline as a screening tool to examine multi-dimensional phenotypes associated with the zebrafish craniofacial skeleton.
Keywords: computational anatomy; cranial morphology; geometric morphometrics; klippel-feil syndrome; osteogenesis imperfecta; osteoporosis; sclerosteosis.
© 2023 Anatomical Society.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Adams, D.C. & Otárola‐Castillo, E. (2013) Geomorph: an R package for the collection and analysis of geometric morphometric shape data. Methods in Ecology and Evolution, 4, 393–399.
-
- Balemans, W. , Ebeling, M. , Patel, N. , Van Hul, E. , Olson, P. , Dioszegi, M. et al. (2001) Increased bone density in sclerosteosis is due to the deficiency of a novel secreted protein (SOST). Human Molecular Genetics, 10, 537–543. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

