Neutralization of Omicron subvariants BA.1 and BA.5 by a booster dose of COVID-19 mRNA vaccine in a Japanese nursing home cohort
- PMID: 36858871
- PMCID: PMC9968608
- DOI: 10.1016/j.vaccine.2023.02.068
Neutralization of Omicron subvariants BA.1 and BA.5 by a booster dose of COVID-19 mRNA vaccine in a Japanese nursing home cohort
Abstract
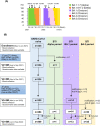
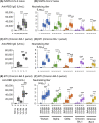
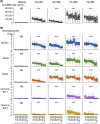
The sustained epidemic of Omicron subvariants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a worldwide concern, and older adults are at high risk. We conducted a prospective cohort study to assess the immunogenicity of COVID-19 mRNA vaccines (BNT162b2 or mRNA-1273) in nursing home residents and staff between May 2021 and December 2022. A total of 335 SARS-CoV-2 naïve individuals, including 141 residents (median age: 88 years) and 194 staff (median age: 44 years) participated. Receptor-binding domain (RBD) and nucleocapsid (N) protein IgG and neutralizing titer (NT) against the Wuhan strain, Alpha and Delta variants, and Omicron BA.1 and BA.5 subvariants were measured in serum samples drawn from participants after the second and third doses of mRNA vaccine using SARS-CoV-2 pseudotyped virus. Breakthrough infection (BTI) was confirmed by a notification of COVID-19 or a positive anti-N IgG result in serum after mRNA vaccination. Fifty-one participants experienced SARS-CoV-2 BTI during the study period. The RBD IgG and NTs against Omicron BA.1 and BA.5 were markedly increased in SARS CoV-2 naïve participants 2 months after the third dose of mRNA vaccine, compared to those 5 months after the second dose, and declined 5 months after the third dose. The decline in RBD IgG and NT against Omicron BA.1 and BA.5 in SARS-CoV-2 naïve participants after the second and the third dose was particularly marked in those aged ≥ 80 years. BTIs during the BA.5 epidemic period, which occurred between 2 and 5 months after the third dose, induced a robust NT against BA.5 even five months after the booster dose vaccination. Further studies are required to assess the sustainability of NTs elicited by Omicron-containing bivalent mRNA booster vaccine in older adults.
Keywords: COVID-19; Neutralizing antibody; Older adults; Omicron subvariants; Pseudotyped virus; mRNA vaccine.
Copyright © 2023 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Immunogenicity of a booster dose of a bivalent (Asp614Gly and omicron BA.4/5 variant) self-amplifying mRNA SARS-CoV-2 booster vaccine versus the BNT162b2 omicron BA.4/5 mRNA vaccine: a randomised phase 3 trial.Lancet Infect Dis. 2025 Mar;25(3):290-300. doi: 10.1016/S1473-3099(24)00565-6. Epub 2024 Oct 23. Lancet Infect Dis. 2025. PMID: 39461355 Clinical Trial.
-
Safety and immunogenicity of the Pfizer/BioNTech SARS-CoV-2 mRNA third booster vaccine dose against the BA.1 and BA.2 Omicron variants.Med. 2022 Jun 10;3(6):406-421.e4. doi: 10.1016/j.medj.2022.04.013. Epub 2022 Apr 26. Med. 2022. PMID: 35815933 Free PMC article.
-
Effects of a booster dose of BNT162b2 on spike-binding antibodies to SARS-CoV-2 Omicron BA.2, BA.3, BA.4 and BA.5 subvariants in infection-naïve and previously-infected individuals.Vaccine. 2023 Jan 23;41(4):879-882. doi: 10.1016/j.vaccine.2022.12.049. Epub 2022 Dec 24. Vaccine. 2023. PMID: 36572601 Free PMC article.
-
Immune evasion of neutralizing antibodies by SARS-CoV-2 Omicron.Cytokine Growth Factor Rev. 2023 Apr;70:13-25. doi: 10.1016/j.cytogfr.2023.03.001. Epub 2023 Mar 5. Cytokine Growth Factor Rev. 2023. PMID: 36948931 Free PMC article. Review.
-
The humoral and cellular immune evasion of SARS-CoV-2 Omicron and sub-lineages.Virol Sin. 2022 Dec;37(6):786-795. doi: 10.1016/j.virs.2022.11.007. Epub 2022 Nov 23. Virol Sin. 2022. PMID: 36427646 Free PMC article. Review.
Cited by
-
Neutralization against Omicron subvariants after BA.5/BF.7 breakthrough infection weakened as virus evolution and aging despite repeated prototype-based vaccination1.Emerg Microbes Infect. 2023 Dec;12(2):2249121. doi: 10.1080/22221751.2023.2249121. Epub 2023 Sep 5. Emerg Microbes Infect. 2023. PMID: 37668156 Free PMC article.
-
Association between sore throat and early immune responses against COVID-19 before and after the emergence of the Omicron variant.Ann Transl Med. 2024 Oct 20;12(5):87. doi: 10.21037/atm-24-36. Epub 2024 Sep 19. Ann Transl Med. 2024. PMID: 39507451 Free PMC article.
-
Antibody and T-Cell Response to Bivalent Booster SARS-CoV-2 Vaccines in People With Compromised Immune Function: COVERALL-3 Study.J Infect Dis. 2024 Oct 16;230(4):e847-e859. doi: 10.1093/infdis/jiae291. J Infect Dis. 2024. PMID: 38848312 Free PMC article. Clinical Trial.
-
Antibody Response to the BA.5 Bivalent Vaccine Shot: a Two-Year Follow-Up Study following Initial COVID-19 mRNA Vaccination.Microbiol Spectr. 2023 Jun 15;11(3):e0131623. doi: 10.1128/spectrum.01316-23. Epub 2023 May 16. Microbiol Spectr. 2023. PMID: 37191496 Free PMC article.
-
COVID-19 mRNA booster vaccination induces robust antibody responses but few adverse events among SARS-CoV-2 naïve nursing home residents.Sci Rep. 2024 Oct 7;14(1):23295. doi: 10.1038/s41598-024-73004-8. Sci Rep. 2024. PMID: 39375365 Free PMC article.
References
-
- The kyodo news service [Internet]. COVID-19 deaths in the nursing facilities account for 14% of overall CIVID-19 deaths [cited 2023 Feb 20]. 47NEWS. Available from: https://47news/4808143.html.
-
- Comas-Herrera A, Marczak J, Byrd W, Lorenz-Dant K, Patel D, Pharoah D (eds.) and LTC covid contributors. LT Covid International living report on COVID-19 and Long-Term Care. Available at: https://ltccovid.org/international-living-report-covid-ltc/.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

