Magnesium oxide-water compounds at megabar pressure and implications on planetary interiors
- PMID: 36859401
- PMCID: PMC9977943
- DOI: 10.1038/s41467-023-36802-8
Magnesium oxide-water compounds at megabar pressure and implications on planetary interiors
Abstract
Magnesium Oxide (MgO) and water (H2O) are abundant in the interior of planets. Their properties, and in particular their interaction, significantly affect the planet interior structure and thermal evolution. Here, using crystal structure predictions and ab initio molecular dynamics simulations, we find that MgO and H2O can react again at ultrahigh pressure, although Mg(OH)2 decomposes at low pressure. The reemergent MgO-H2O compounds are: Mg2O3H2 above 400 GPa, MgO3H4 above 600 GPa, and MgO4H6 in the pressure range of 270-600 GPa. Importantly, MgO4H6 contains 57.3 wt % of water, which is a much higher water content than any reported hydrous mineral. Our results suggest that a substantial amount of water can be stored in MgO rock in the deep interiors of Earth to Neptune mass planets. Based on molecular dynamics simulations we show that these three compounds exhibit superionic behavior at the pressure-temperature conditions as in the interiors of Uranus and Neptune. Moreover, the water-rich compound MgO4H6 could be stable inside the early Earth and therefore may serve as a possible early Earth water reservoir. Our findings, in the poorly explored megabar pressure regime, provide constraints for interior and evolution models of wet planets in our solar system and beyond.
© 2023. The Author(s).
Conflict of interest statement
C.J.P. is an author of the CASTEP code, and receives royalty payments from its commercial sales by Dassault Systèmes. The remaining authors declare no competing interests.
Figures



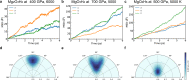
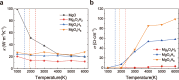

References
-
- Rogers LA, Seager S. A framework for quantifying the degeneracies of exoplanet interior compositions. Astrophys. J. 2010;712:974–991. doi: 10.1088/0004-637X/712/2/974. - DOI
-
- Redmer R, Mattsson TR, Nettelmann N, French M. The phase diagram of water and the magnetic fields of Uranus and Neptune. Icarus. 2011;211:798–803. doi: 10.1016/j.icarus.2010.08.008. - DOI
-
- Nettelmann N, Helled R, Fortney JJ, Redmer R. New indication for a dichotomy in the interior structure of Uranus and Neptune from the application of modified shape and rotation data. Planet. Space Sci. 2013;77:143–151. doi: 10.1016/j.pss.2012.06.019. - DOI
-
- Noack L, et al. Water-rich planets: How habitable is a water layer deeper than on Earth? Icarus. 2016;277:215–236. doi: 10.1016/j.icarus.2016.05.009. - DOI
-
- Dorn C, et al. A generalized Bayesian inference method for constraining the interiors of super Earths and sub-Neptunes. Astron. Astrophys. 2017;597:1–16. doi: 10.1051/0004-6361/201628708. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

