Cardiogenic control of affective behavioural state
- PMID: 36859543
- PMCID: PMC9995271
- DOI: 10.1038/s41586-023-05748-8
Cardiogenic control of affective behavioural state
Abstract
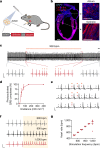
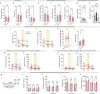
Emotional states influence bodily physiology, as exemplified in the top-down process by which anxiety causes faster beating of the heart1-3. However, whether an increased heart rate might itself induce anxiety or fear responses is unclear3-8. Physiological theories of emotion, proposed over a century ago, have considered that in general, there could be an important and even dominant flow of information from the body to the brain9. Here, to formally test this idea, we developed a noninvasive optogenetic pacemaker for precise, cell-type-specific control of cardiac rhythms of up to 900 beats per minute in freely moving mice, enabled by a wearable micro-LED harness and the systemic viral delivery of a potent pump-like channelrhodopsin. We found that optically evoked tachycardia potently enhanced anxiety-like behaviour, but crucially only in risky contexts, indicating that both central (brain) and peripheral (body) processes may be involved in the development of emotional states. To identify potential mechanisms, we used whole-brain activity screening and electrophysiology to find brain regions that were activated by imposed cardiac rhythms. We identified the posterior insular cortex as a potential mediator of bottom-up cardiac interoceptive processing, and found that optogenetic inhibition of this brain region attenuated the anxiety-like behaviour that was induced by optical cardiac pacing. Together, these findings reveal that cells of both the body and the brain must be considered together to understand the origins of emotional or affective states. More broadly, our results define a generalizable approach for noninvasive, temporally precise functional investigations of joint organism-wide interactions among targeted cells during behaviour.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures











Comment in
-
How an anxious heart talks to the brain.Nature. 2023 Mar;615(7951):217-218. doi: 10.1038/d41586-023-00502-6. Nature. 2023. PMID: 36859663 No abstract available.
-
Optically induced tachycardia evokes anxiety in risky settings.Nat Rev Cardiol. 2023 May;20(5):283. doi: 10.1038/s41569-023-00858-2. Nat Rev Cardiol. 2023. PMID: 36932154 No abstract available.
-
The cardiac origin of anxiety.Nat Cardiovasc Res. 2023 Apr;2(4):339. doi: 10.1038/s44161-023-00265-3. Nat Cardiovasc Res. 2023. PMID: 39195954 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

