The CRAFITY score can identify patients with hepatocellular carcinoma showing poor response to treatment with atezolizumab and bevacizumab
- PMID: 36860264
- PMCID: PMC9944522
- DOI: 10.21037/hbsn-22-280
The CRAFITY score can identify patients with hepatocellular carcinoma showing poor response to treatment with atezolizumab and bevacizumab
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://hbsn.amegroups.com/article/view/10.21037/hbsn-22-280/coif). FvB reported honoraria from Roche, Eisai, Ipsen, MSD, Astra Zeneca and Gilead Sciences; support for attending meetings and/or travel from Gilead Sciences, Eisai; equipment, materials, drugs, medical writing, gifts or other services from Siemens, Diasorin, and Roche, and FvB was on the Data Safety Monitoring Board or Advisory Board of Janssen. TB reported grants or contracts from Abbvie, BMS, Gilead, MSD/Merck, Humedics, Intercept, Merz, Novartis and Sequana Medical; reported consulting fees from Abbvie, Alexion, Bayer, Gilead, Eisai, GSK, Intercept, Ipsen, Janssen, MSD/Merck, Novartis, Roche, Sequana Medical, and Shionogi; reported honoraria from Abbvie, Alexion, Bayer, Gilead, Eisai, Intercept, Ipsen, Janssen, MedUpdate GmbH, MSD/Merck, Novartis, and Sequana Medica; reported support for attending meetings and/or travel from Gilead, Abbvie, Intercept, and Janssen. DS reported honoraria from Astellas, BTG, and Novartis; reported support for attending meetings and/or travel from Gilead, Abbvie, Intercept, Janssen, and Johnson & Johnson and DS was on the Data Safety Monitoring Board or Advisory Board of BTG, SIRTEX, and Olympus. The other authors have no conflicts of interest to declare.
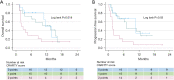
Figures
Comment on
-
Immunotherapy for hepatocellular carcinoma: a "CRAFITY" approach to patient stratification.Hepatobiliary Surg Nutr. 2022 Apr;11(2):327-329. doi: 10.21037/hbsn-2021-32. Hepatobiliary Surg Nutr. 2022. PMID: 35464263 Free PMC article. No abstract available.
References
-
- FDA approves atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma. FDA. Updated June 1, 2020. Accessed April 24, 2022. Available online: https://www.bit.ly/3MsBuDd
-
- Summary of opinion (post-authorisation) EMA/CHMP/488242/2020. EMA CHMP. 2020. Available online: https://www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-summ...
Publication types
LinkOut - more resources
Full Text Sources