Inhibitory effect of lactobacilli supernatants on biofilm and filamentation of Candida albicans, Candida tropicalis, and Candida parapsilosis
- PMID: 36860488
- PMCID: PMC9969145
- DOI: 10.3389/fmicb.2023.1105949
Inhibitory effect of lactobacilli supernatants on biofilm and filamentation of Candida albicans, Candida tropicalis, and Candida parapsilosis
Abstract
Introduction: Probiotic Lactobacillus strains had been investigated for the potential to protect against infection caused by the major fungal pathogen of human, Candida albicans. Besides antifungal activity, lactobacilli demonstrated a promising inhibitory effect on biofilm formation and filamentation of C. albicans. On the other hand, two commonly isolated non-albicans Candida species, C. tropicalis and C. parapsilosis, have similar characteristics in filamentation and biofilm formation with C. albicans. However, there is scant information of the effect of lactobacilli on the two species.
Methods: In this study, biofilm inhibitory effects of L. rhamnosus ATCC 53103, L. plantarum ATCC 8014, and L. acidophilus ATCC 4356 were tested on the reference strain C. albicans SC5314 and six bloodstream isolated clinical strains, two each of C. albicans, C. tropicalis, and C. parapsilosis.
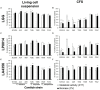
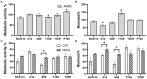
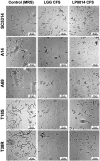
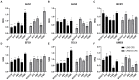
Results and discussion: Cell-free culture supernatants (CFSs) of L. rhamnosus and L. plantarum significantly inhibited in vitro biofilm growth of C. albicans and C. tropicalis. L. acidophilus, conversely, had little effect on C. albicans and C. tropicalis but was more effective on inhibiting C. parapsilosis biofilms. Neutralized L. rhamnosus CFS at pH 7 retained the inhibitory effect, suggesting that exometabolites other than lactic acid produced by the Lactobacillus strain might be accounted for the effect. Furthermore, we evaluated the inhibitory effects of L. rhamnosus and L. plantarum CFSs on the filamentation of C. albicans and C. tropicalis strains. Significantly less Candida filaments were observed after co-incubating with CFSs under hyphae-inducing conditions. Expressions of six biofilm-related genes (ALS1, ALS3, BCR1, EFG1, TEC1, and UME6 in C. albicans and corresponding orthologs in C. tropicalis) in biofilms co-incubated with CFSs were analyzed using quantitative real-time PCR. When compared to untreated control, the expressions of ALS1, ALS3, EFG1, and TEC1 genes were downregulated in C. albicans biofilm. In C. tropicalis biofilms, ALS3 and UME6 were downregulated while TEC1 was upregulated. Taken together, the L. rhamnosus and L. plantarum strains demonstrated an inhibitory effect, which is likely mediated by the metabolites secreted into culture medium, on filamentation and biofilm formation of C. albicans and C. tropicalis. Our finding suggested an alternative to antifungals for controlling Candida biofilm.
Keywords: Candida; Lactobacillus; biofilm; filamentation; gene expression; non-albicans Candida species.
Copyright © 2023 Poon and Hui.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Probiotic lactobacilli inhibit early stages of Candida albicans biofilm development by reducing their growth, cell adhesion, and filamentation.Appl Microbiol Biotechnol. 2016 Jul;100(14):6415-6426. doi: 10.1007/s00253-016-7527-3. Epub 2016 Apr 18. Appl Microbiol Biotechnol. 2016. PMID: 27087525
-
Inhibitory effect of probiotic lactobacilli supernatants on single and mixed non-albicans Candida species biofilm.Arch Oral Biol. 2018 Jan;85:40-45. doi: 10.1016/j.archoralbio.2017.10.002. Epub 2017 Oct 6. Arch Oral Biol. 2018. PMID: 29031236
-
Lactobacillus acidophilus, L. plantarum, L. rhamnosus, and L. reuteri Cell-Free Supernatants Inhibit Candida parapsilosis Pathogenic Potential upon Infection of Vaginal Epithelial Cells Monolayer and in a Transwell Coculture System In Vitro.Microbiol Spectr. 2022 Jun 29;10(3):e0269621. doi: 10.1128/spectrum.02696-21. Epub 2022 May 2. Microbiol Spectr. 2022. PMID: 35499353 Free PMC article.
-
Filamentation Is Associated with Reduced Pathogenicity of Multiple Non-albicans Candida Species.mSphere. 2019 Oct 16;4(5):e00656-19. doi: 10.1128/mSphere.00656-19. mSphere. 2019. PMID: 31619502 Free PMC article.
-
Culture Supernatants of Lactobacillus gasseri and L. crispatus Inhibit Candida albicans Biofilm Formation and Adhesion to HeLa Cells.Mycopathologia. 2018 Aug;183(4):691-700. doi: 10.1007/s11046-018-0259-4. Epub 2018 Mar 30. Mycopathologia. 2018. PMID: 29603066
Cited by
-
Baicalein as a potent antifungal agent against Candida albicans: synergy with fluconazole and sustainable production through probiotic-mediated bioconversion.Front Microbiol. 2025 Feb 25;16:1562103. doi: 10.3389/fmicb.2025.1562103. eCollection 2025. Front Microbiol. 2025. PMID: 40071210 Free PMC article.
-
Effects of Thymus daenensis Essential Oil-loaded chitosan Nanoparticles on BCR1 Gene Expression in Candida Parapsilosis.Arch Razi Inst. 2024 Oct 31;79(5):973-980. doi: 10.32592/ARI.2024.79.5.973. eCollection 2024 Oct. Arch Razi Inst. 2024. PMID: 40292051 Free PMC article.
-
A Re-Purposing Strategy: Sub-Lethal Concentrations of an Eicosanoid Derived from the Omega-3-Polyunsaturated Fatty Acid Resolvin D1 Affect Dual Species Biofilms.Int J Mol Sci. 2023 Aug 17;24(16):12876. doi: 10.3390/ijms241612876. Int J Mol Sci. 2023. PMID: 37629056 Free PMC article.
-
Biomimetic Antifungal Materials: Countering the Challenge of Multidrug-Resistant Fungi.Biomimetics (Basel). 2024 Jul 12;9(7):425. doi: 10.3390/biomimetics9070425. Biomimetics (Basel). 2024. PMID: 39056866 Free PMC article. Review.
-
Vaginal Candida albicans infections: host-pathogen-microbiome interactions.FEMS Microbiol Rev. 2025 Jan 14;49:fuaf013. doi: 10.1093/femsre/fuaf013. FEMS Microbiol Rev. 2025. PMID: 40347186 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

