Trehalose-6-phosphate synthase regulates chitin synthesis in Mythimna separata
- PMID: 36860522
- PMCID: PMC9968958
- DOI: 10.3389/fphys.2023.1109661
Trehalose-6-phosphate synthase regulates chitin synthesis in Mythimna separata
Abstract
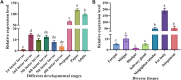
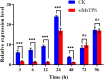
Trehalose is a substrate for the chitin synthesis pathway in insects. Thus, it directly affects chitin synthesis and metabolism. Trehalose-6-phosphate synthase (TPS) is a crucial enzyme in the trehalose synthesis pathway in insects, but its functions in Mythimna separata remain unclear. In this study, a TPS-encoding sequence in M. separata (MsTPS) was cloned and characterized. Its expression patterns at different developmental stages and in diverse tissues were investigated. The results indicated that MsTPS was expressed at all analyzed developmental stages, with peak expression levels in the pupal stage. Moreover, MsTPS was expressed in the foregut, midgut, hindgut, fat body, salivary gland, Malpighian tubules, and integument, with the highest expression levels in the fat body. The inhibition of MsTPS expression via RNA interference (RNAi) resulted in significant decreases in the trehalose content and TPS activity. It also resulted in significant changes in Chitin synthase (MsCHSA and MsCHSB) expression, and significantly decrease the chitin content in the midgut and integument of M. separata. Additionally, the silencing of MsTPS was associated with a significant decrease in M. separata weight, larval feed intake, and ability to utilize food. It also induced abnormal phenotypic changes and increased the M. separata mortality and malformation rates. Hence, MsTPS is important for M. separata chitin synthesis. The results of this study also suggest RNAi technology may be useful for enhancing the methods used to control M. separata infestations.
Keywords: Mythimna separata; RNAi; chitin; trehalose; trehalose-6-phosphate synthase.
Copyright © 2023 Yang, Cui, Zhao, Zhang, Hu and Fan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Arakane Y., Muthukrishnan S., Kramer K. J., Specht C. A., Tomoyasu Y., Lorenzen M. D., et al. (2005). The Tribolium chitin synthase genes TcCHS1 and TcCHS2 are specialized for synthesis of epidermal cuticle and midgut peritrophic matrix. Insect Mol. Biol. 14 (5), 453–463. 10.1111/j.1365-2583.2005.00576.x - DOI - PubMed
LinkOut - more resources
Full Text Sources

