Vitrimer ionogels towards sustainable solid-state electrolytes
- PMID: 36860526
- PMCID: PMC9969235
- DOI: 10.1039/d2ra06829j
Vitrimer ionogels towards sustainable solid-state electrolytes
Abstract
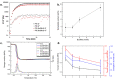
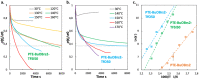
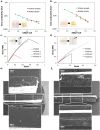
The growing demand for flexible, stretchable, and wearable devices has boosted the development of ionogels used as polymer electrolytes. Developing healable ionogels based on vitrimer chemistry is a promising approach to improve their lifetimes as these materials are usually subjected to repeated deformation during functioning and are susceptible to damage. In this work, we reported in the first place the preparation of polythioether vitrimer networks based on the not extensively studied associative S-transalkylation exchange reaction using thiol-ene Michael addition. Thanks to the exchange reaction of sulfonium salts with thioether nucleophiles, these materials demonstrated vitrimer properties such as healing and stress relaxation. The fabrication of dynamic polythioether ionogels was then demonstrated by loading 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide or 1-ethyl-3-methylimidazolium trifluoromethanesulfonate (EMIM triflate) within the polymer network. The resulting ionogels exhibited Young's modulus of 0.9 MPa and ionic conductivities in the order of 10-4 S cm-1 at room temperature. It has been found that adding ionic liquids (ILs) changes the dynamic properties of the systems, most likely due to a dilution effect of the dynamic functions by the IL but also due to a screening effect of the alkyl sulfonium OBrs-couple by the ions of the IL itself. To the best of our knowledge, these are the first vitrimer ionogels based on an S-transalkylation exchange reaction. While the addition of ILs resulted in less efficient dynamic healing at a given temperature, these ionogels can provide materials with more dimensional stability at application temperatures and can potentially pave the way for the development of tunable dynamic ionogels for flexible electronics with a longer lifespan.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous

