Modified magnetic chitosan materials for heavy metal adsorption: a review
- PMID: 36860541
- PMCID: PMC9969337
- DOI: 10.1039/d2ra07112f
Modified magnetic chitosan materials for heavy metal adsorption: a review
Abstract
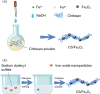
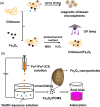
Magnetic chitosan materials have the characteristics of both chitosan and magnetic particle nuclei, showing the characteristics of easy separation and recovery, strong adsorption capacity and high mechanical strength, and have received extensive attention in adsorption, especially in the treatment of heavy metal ions. In order to further improve its performance, many studies have modified magnetic chitosan materials. This review discusses the strategies for the preparation of magnetic chitosan using coprecipitation, crosslinking, and other methods in detail. Besides, this review mainly summarizes the application of modified magnetic chitosan materials in the removal of heavy metal ions in wastewater in recent years. Finally, this review also discusses the adsorption mechanism, and puts forward the prospect of the future development of magnetic chitosan in wastewater treatment.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures













References
-
- Vatanpour N. Feizy J. Talouki H. H. Es’haghi Z. Scesi L. Malvandi A. M. Chemosphere. 2020;245:125639. - PubMed
-
- Rana K. Kumar M. Kumar A. Water, Air, Soil Pollut. 2020;231:450.
-
- Adimalla N. Chen J. Qian H. Ecotoxicol. Environ. Saf. 2020;194:110406. - PubMed
-
- Kumar A. Cabral-Pinto M. Kumar M. Dinis P. A. Appl. Sci. 2020;10:7078.
Publication types
LinkOut - more resources
Full Text Sources

