Glycine receptor autoantibody binding to the extracellular domain is independent from receptor glycosylation
- PMID: 36860666
- PMCID: PMC9969106
- DOI: 10.3389/fnmol.2023.1089101
Glycine receptor autoantibody binding to the extracellular domain is independent from receptor glycosylation
Abstract
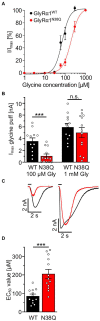
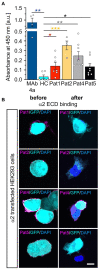
Glycine receptor (GlyR) autoantibodies are associated with stiff-person syndrome and the life-threatening progressive encephalomyelitis with rigidity and myoclonus in children and adults. Patient histories show variability in symptoms and responses to therapeutic treatments. A better understanding of the autoantibody pathology is required to develop improved therapeutic strategies. So far, the underlying molecular pathomechanisms include enhanced receptor internalization and direct receptor blocking altering GlyR function. A common epitope of autoantibodies against the GlyRα1 has been previously defined to residues 1A-33G at the N-terminus of the mature GlyR extracellular domain. However, if other autoantibody binding sites exist or additional GlyR residues are involved in autoantibody binding is yet unknown. The present study investigates the importance of receptor glycosylation for binding of anti-GlyR autoantibodies. The glycine receptor α1 harbors only one glycosylation site at the amino acid residue asparagine 38 localized in close vicinity to the identified common autoantibody epitope. First, non-glycosylated GlyRs were characterized using protein biochemical approaches as well as electrophysiological recordings and molecular modeling. Molecular modeling of non-glycosylated GlyRα1 did not show major structural alterations. Moreover, non-glycosylation of the GlyRα1N38Q did not prevent the receptor from surface expression. At the functional level, the non-glycosylated GlyR demonstrated reduced glycine potency, but patient GlyR autoantibodies still bound to the surface-expressed non-glycosylated receptor protein in living cells. Efficient adsorption of GlyR autoantibodies from patient samples was possible by binding to native glycosylated and non-glycosylated GlyRα1 expressed in living not fixed transfected HEK293 cells. Binding of patient-derived GlyR autoantibodies to the non-glycosylated GlyRα1 offered the possibility to use purified non-glycosylated GlyR extracellular domain constructs coated on ELISA plates and use them as a fast screening readout for the presence of GlyR autoantibodies in patient serum samples. Following successful adsorption of patient autoantibodies by GlyR ECDs, binding to primary motoneurons and transfected cells was absent. Our results indicate that the glycine receptor autoantibody binding is independent of the receptor's glycosylation state. Purified non-glycosylated receptor domains harbouring the autoantibody epitope thus provide, an additional reliable experimental tool besides binding to native receptors in cell-based assays for detection of autoantibody presence in patient sera.
Keywords: adsorption; autoantibodies; extracellular domain; glycine receptor; glycosylation.
Copyright © 2023 Rauschenberger, Piro, Kasaragod, Hörlin, Eckes, Kluck, Schindelin, Meinck, Wickel, Geis, Tüzün, Doppler, Sommer and Villmann.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Glycine Receptor β-Targeting Autoantibodies Contribute to the Pathology of Autoimmune Diseases.Neurol Neuroimmunol Neuroinflamm. 2024 Mar;11(2):e200187. doi: 10.1212/NXI.0000000000200187. Epub 2024 Jan 12. Neurol Neuroimmunol Neuroinflamm. 2024. PMID: 38215349 Free PMC article.
-
Impaired Presynaptic Function Contributes Significantly to the Pathology of Glycine Receptor Autoantibodies.Neurol Neuroimmunol Neuroinflamm. 2025 Mar;12(2):e200364. doi: 10.1212/NXI.0000000000200364. Epub 2025 Jan 16. Neurol Neuroimmunol Neuroinflamm. 2025. PMID: 39819053 Free PMC article.
-
Glycine Receptor Autoantibodies Impair Receptor Function and Induce Motor Dysfunction.Ann Neurol. 2020 Sep;88(3):544-561. doi: 10.1002/ana.25832. Epub 2020 Jul 20. Ann Neurol. 2020. PMID: 32588476
-
Impaired Glycine Receptor Trafficking in Neurological Diseases.Front Mol Neurosci. 2018 Aug 21;11:291. doi: 10.3389/fnmol.2018.00291. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30186111 Free PMC article. Review.
-
Redefining progressive encephalomyelitis with rigidity and myoclonus after the discovery of antibodies to glycine receptors.Curr Opin Neurol. 2017 Jun;30(3):310-316. doi: 10.1097/WCO.0000000000000450. Curr Opin Neurol. 2017. PMID: 28306573 Review.
Cited by
-
Glycine Receptor β-Targeting Autoantibodies Contribute to the Pathology of Autoimmune Diseases.Neurol Neuroimmunol Neuroinflamm. 2024 Mar;11(2):e200187. doi: 10.1212/NXI.0000000000200187. Epub 2024 Jan 12. Neurol Neuroimmunol Neuroinflamm. 2024. PMID: 38215349 Free PMC article.
References
-
- Atak S., Langlhofer G., Schaefer N., Kessler D., Meiselbach H., Delto C., et al. . (2015). Disturbances of ligand potency and enhanced degradation of the human glycine receptor at affected positions G160 and T162 originally identified in patients suffering from Hyperekplexia. Front. Mol. Neurosci. 8:79. doi: 10.3389/fnmol.2015.00079 - DOI - PMC - PubMed
-
- Breitinger U., Breitinger H. G., Bauer F., Fahmy K., Glockenhammer D., Becker C. M. (2004). Conserved high affinity ligand binding and membrane association in the native and refolded extracellular domain of the human glycine receptor alpha1-subunit. J. Biol. Chem. 279, 1627–1636. doi: 10.1074/jbc.M303811200, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources

