C3a-C3aR signaling is a novel modulator of skeletal homeostasis
- PMID: 36860797
- PMCID: PMC9969257
- DOI: 10.1016/j.bonr.2023.101662
C3a-C3aR signaling is a novel modulator of skeletal homeostasis
Abstract
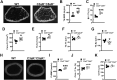
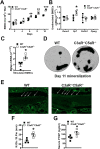
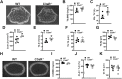
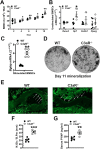
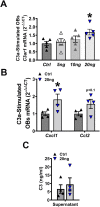
Osteoimmune studies have identified complement signaling as an important regulator of the skeleton. Specifically, complement anaphylatoxin receptors (i.e., C3aR, C5aR) are expressed on osteoblasts and osteoclasts, implying that C3a and/or C5a may be candidate mediators of skeletal homeostasis. The study aimed to determine how complement signaling influences bone modeling/remodeling in the young skeleton. Female C57BL/6J C3aR-/-C5aR-/- vs. wildtype and C3aR-/- vs. wildtype mice were examined at age 10 weeks. Trabecular and cortical bone parameters were analyzed by micro-CT. In situ osteoblast and osteoclast outcomes were determined by histomorphometry. Osteoblast and osteoclast precursors were assessed in vitro. C3aR-/-C5aR-/- mice displayed an increased trabecular bone phenotype at age 10 weeks. In vitro studies revealed C3aR-/-C5aR-/- vs. wildtype cultures had less bone-resorbing osteoclasts and increased bone-forming osteoblasts, which were validated in vivo. To determine whether C3aR alone was critical for the enhanced skeletal outcomes, wildtype vs. C3aR-/- mice were evaluated for osseous tissue outcomes. Paralleling skeletal findings in C3aR-/-C5aR-/- mice, C3aR-/- vs. wildtype mice had an enhanced trabecular bone volume fraction, which was attributed to increased trabecular number. There was elevated osteoblast activity and suppressed osteoclastic cells in C3aR-/- vs. wildtype mice. Furthermore, primary osteoblasts derived from wildtype mice were stimulated with exogenous C3a, which more profoundly upregulated C3ar1 and the pro-osteoclastic chemokine Cxcl1. This study introduces the C3a/C3aR signaling axis as a novel regulator of the young skeleton.
Keywords: Complement; Osteoblast; Osteoclast; Osteoimmunology.
Published by Elsevier Inc.
Conflict of interest statement
All authors, Megan B. Kuhn, Hayden S. Vandenburg, Andrew J. Reynolds, Matthew D. Carson, Amy J. Warner, Amanda C. LaRue, Chad M. Novince, and Jessica D. Hathaway-Schrader, declare no conflicts of interest.
Figures


References
-
- Andrades J.A., Nimni M.E., Becerra J., Eisenstein R., Davis M., Sorgente N. Complement proteins are present in developing endochondral bone and may mediate cartilage cell death and vascularization. Exp. Cell Res. 1996;227(2):208–213. - PubMed
-
- Baxter-Jones A.D., Faulkner R.A., Forwood M.R., Mirwald R.L., Bailey D.A. Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J. Bone Miner. Res. 2011;26(8):1729–1739. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

