Is Disrupted Mitophagy a Central Player to Parkinson's Disease Pathology?
- PMID: 36860818
- PMCID: PMC9969326
- DOI: 10.7759/cureus.35458
Is Disrupted Mitophagy a Central Player to Parkinson's Disease Pathology?
Abstract
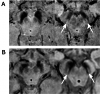
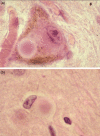
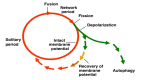
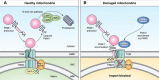
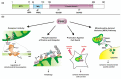
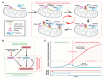
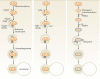
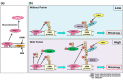
Whilst the pathophysiology at a cellular level has been defined, the cause of Parkinson's disease (PD) remains poorly understood. This neurodegenerative disorder is associated with impaired dopamine transmission in the substantia nigra, and protein accumulations known as Lewy bodies are visible in affected neurons. Cell culture models of PD have indicated impaired mitochondrial function, so the focus of this paper is on the quality control processes involved in and around mitochondria. Mitochondrial autophagy (mitophagy) is the process through which defective mitochondria are removed from the cell by internalisation into autophagosomes which fuse with a lysosome. This process involves many proteins, notably including PINK1 and parkin, both of which are known to be coded on genes associated with PD. Normally in healthy individuals, PINK1 associates with the outer mitochondrial membrane, which then recruits parkin, activating it to attach ubiquitin proteins to the mitochondrial membrane. PINK1, parkin, and ubiquitin cooperate to form a positive feedback system which accelerates the deposition of ubiquitin on dysfunctional mitochondria, resulting in mitophagy. However, in hereditary PD, the genes encoding PINK1 and parkin are mutated, resulting in proteins that are less efficient at removing poorly performing mitochondria, leaving cells more vulnerable to oxidative stress and ubiquitinated inclusion bodies, such as Lewy bodies. Current research that looks into the connection between mitophagy and PD is promising, already yielding potentially therapeutic compounds; until now, pharmacological support for the mitophagy process has not been part of the therapeutic arsenal. Continued research in this area is warranted.
Keywords: lewy body; mitophagy; parkin; parkinson's disease; pink1.
Copyright © 2023, Ko et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
N-degron-mediated degradation and regulation of mitochondrial PINK1 kinase.Curr Genet. 2020 Aug;66(4):693-701. doi: 10.1007/s00294-020-01062-2. Epub 2020 Mar 10. Curr Genet. 2020. PMID: 32157382 Review.
-
Nix restores mitophagy and mitochondrial function to protect against PINK1/Parkin-related Parkinson's disease.Sci Rep. 2017 Mar 10;7:44373. doi: 10.1038/srep44373. Sci Rep. 2017. PMID: 28281653 Free PMC article.
-
Rab11 regulates mitophagy signaling pathway of Parkin and Pink1 in the Drosophila model of Parkinson's disease.Biochem Biophys Res Commun. 2022 Oct 20;626:175-186. doi: 10.1016/j.bbrc.2022.08.027. Epub 2022 Aug 12. Biochem Biophys Res Commun. 2022. PMID: 35994827
-
Effects of electroacupuncture on mitophagy mediated by SIRT3/PINK1/Parkin pathway in Parkinson's disease mice.Zhen Ci Yan Jiu. 2024 Mar 25;49(3):221-230. doi: 10.13702/j.1000-0607.20230654. Zhen Ci Yan Jiu. 2024. PMID: 38500318 Chinese, English.
-
The Role of PTEN-L in Modulating PINK1-Parkin-Mediated Mitophagy.Neurotox Res. 2022 Aug;40(4):1103-1114. doi: 10.1007/s12640-022-00475-w. Epub 2022 Jun 14. Neurotox Res. 2022. PMID: 35699891 Review.
Cited by
-
Targets to Search for New Pharmacological Treatment in Idiopathic Parkinson's Disease According to the Single-Neuron Degeneration Model.Biomolecules. 2024 Jun 8;14(6):673. doi: 10.3390/biom14060673. Biomolecules. 2024. PMID: 38927076 Free PMC article. Review.
-
Methamphetamine Increases Tubulo-Vesicular Areas While Dissipating Proteins from Vesicles Involved in Cell Clearance.Int J Mol Sci. 2024 Sep 4;25(17):9601. doi: 10.3390/ijms25179601. Int J Mol Sci. 2024. PMID: 39273545 Free PMC article.
-
Mitophagy and cGAS-STING crosstalk in neuroinflammation.Acta Pharm Sin B. 2024 Aug;14(8):3327-3361. doi: 10.1016/j.apsb.2024.05.012. Epub 2024 May 13. Acta Pharm Sin B. 2024. PMID: 39220869 Free PMC article. Review.
-
CLEC16A-An Emerging Master Regulator of Autoimmunity and Neurodegeneration.Int J Mol Sci. 2023 May 4;24(9):8224. doi: 10.3390/ijms24098224. Int J Mol Sci. 2023. PMID: 37175930 Free PMC article. Review.
-
Neurodegeneration models in Parkinson's disease: cellular and molecular paths to neuron death.Behav Brain Funct. 2025 May 31;21(1):14. doi: 10.1186/s12993-025-00279-w. Behav Brain Funct. 2025. PMID: 40450319 Free PMC article. Review.
References
-
- Parkinson's disease: genetics and pathogenesis. Shulman JM, De Jager PL, Feany MB. Annu Rev Pathol. 2011;6:193–222. - PubMed
-
- World Health Organization. Neurological Disorders: Public Health Challenges. Geneva, WHO. [ Dec; 2022 ]. 2006. https://www.who.int/publications/i/item/9789241563369 https://www.who.int/publications/i/item/9789241563369
-
- The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Pringsheim T, Jette N, Frolkis A, Steeves TD. Mov Disord. 2014;29:1583–1590. - PubMed
-
- Parkinson's UK, 2013 2013. Parkinson’s and the NHS in England: the cost of poor care. [ Dec; 2022 ]. 2013. http://www.innovationagencyexchange.org.uk/sites/default/files/Parkinson... http://www.innovationagencyexchange.org.uk/sites/default/files/Parkinson...
-
- Gowers WR. London: J. & A. Churchill; 1886. A Manual of Diseases of the Nervous System.
Publication types
LinkOut - more resources
Full Text Sources
