Persistent memory despite rapid contraction of circulating T Cell responses to SARS-CoV-2 mRNA vaccination
- PMID: 36860850
- PMCID: PMC9968837
- DOI: 10.3389/fimmu.2023.1100594
Persistent memory despite rapid contraction of circulating T Cell responses to SARS-CoV-2 mRNA vaccination
Abstract
Introduction: While antibodies raised by SARS-CoV-2 mRNA vaccines have had compromised efficacy to prevent breakthrough infections due to both limited durability and spike sequence variation, the vaccines have remained highly protective against severe illness. This protection is mediated through cellular immunity, particularly CD8+ T cells, and lasts at least a few months. Although several studies have documented rapidly waning levels of vaccine-elicited antibodies, the kinetics of T cell responses have not been well defined.
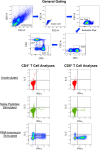
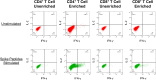
Methods: Interferon (IFN)-γ enzyme-linked immunosorbent spot (ELISpot) assay and intracellular cytokine staining (ICS) were utilized to assess cellular immune responses (in isolated CD8+ T cells or whole peripheral blood mononuclear cells, PBMCs) to pooled peptides spanning spike. ELISA was performed to quantitate serum antibodies against the spike receptor binding domain (RBD).
Results: In two persons receiving primary vaccination, tightly serially evaluated frequencies of anti-spike CD8+ T cells using ELISpot assays revealed strikingly short-lived responses, peaking after about 10 days and becoming undetectable by about 20 days after each dose. This pattern was also observed in cross-sectional analyses of persons after the first and second doses during primary vaccination with mRNA vaccines. In contrast, cross-sectional analysis of COVID-19-recovered persons using the same assay showed persisting responses in most persons through 45 days after symptom onset. Cross-sectional analysis using IFN-γ ICS of PBMCs from persons 13 to 235 days after mRNA vaccination also demonstrated undetectable CD8+ T cells against spike soon after vaccination, and extended the observation to include CD4+ T cells. However, ICS analyses of the same PBMCs after culturing with the mRNA-1273 vaccine in vitro showed CD4+ and CD8+ T cell responses that were readily detectable in most persons out to 235 days after vaccination.
Discussion: Overall, we find that detection of spike-targeted responses from mRNA vaccines using typical IFN-γ assays is remarkably transient, which may be a function of the mRNA vaccine platform and an intrinsic property of the spike protein as an immune target. However, robust memory, as demonstrated by capacity for rapid expansion of T cells responding to spike, is maintained at least several months after vaccination. This is consistent with the clinical observation of vaccine protection from severe illness lasting months. The level of such memory responsiveness required for clinical protection remains to be defined.
Keywords: SARS-CoV-2; SARS-CoV-2 mRNA vaccines; T cell memory; T cells; cellular immunity; elispot; intracellular cytokine staining.
Copyright © 2023 Taus, Hofmann, Ibarrondo, Gong, Hausner, Fulcher, Krogstad, Kitchen, Ferbas, Tobin, Rimoin, Aldrovandi and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures



Similar articles
-
Predominantly defective CD8+ T cell immunity to SARS-CoV-2 mRNA vaccination in lung transplant recipients.J Transl Med. 2023 Jun 8;21(1):374. doi: 10.1186/s12967-023-04234-z. J Transl Med. 2023. PMID: 37291575 Free PMC article.
-
Dominant CD8+ T Cell Nucleocapsid Targeting in SARS-CoV-2 Infection and Broad Spike Targeting From Vaccination.Front Immunol. 2022 Feb 22;13:835830. doi: 10.3389/fimmu.2022.835830. eCollection 2022. Front Immunol. 2022. PMID: 35273611 Free PMC article.
-
Adaptive immune responses and cytokine immune profiles in humans following prime and boost vaccination with the SARS-CoV-2 CoronaVac vaccine.Virol J. 2022 Dec 22;19(1):223. doi: 10.1186/s12985-022-01957-1. Virol J. 2022. PMID: 36550578 Free PMC article.
-
Durable CD8 T Cell Memory against SARS-CoV-2 by Prime/Boost and Multi-Dose Vaccination: Considerations on Inter-Dose Time Intervals.Int J Mol Sci. 2022 Nov 19;23(22):14367. doi: 10.3390/ijms232214367. Int J Mol Sci. 2022. PMID: 36430845 Free PMC article. Review.
-
Defending against SARS-CoV-2: The T cell perspective.Front Immunol. 2023 Jan 27;14:1107803. doi: 10.3389/fimmu.2023.1107803. eCollection 2023. Front Immunol. 2023. PMID: 36776863 Free PMC article. Review.
Cited by
-
Development of an ELISPOT assay for numerating IFN-γ-secreting T cells in chicken using novel monoclonal antibodies.Poult Sci. 2025 Jul 10;104(10):105524. doi: 10.1016/j.psj.2025.105524. Online ahead of print. Poult Sci. 2025. PMID: 40669239 Free PMC article.
-
Five doses of the mRNA vaccination potentially suppress ancestral-strain stimulated SARS-CoV2-specific cellular immunity: a cohort study from the Fukushima vaccination community survey, Japan.Front Immunol. 2023 Aug 16;14:1240425. doi: 10.3389/fimmu.2023.1240425. eCollection 2023. Front Immunol. 2023. PMID: 37662950 Free PMC article.
-
The molecular mechanisms of CD8+ T cell responses to SARS-CoV-2 infection mediated by TCR-pMHC interactions.Front Immunol. 2024 Oct 10;15:1468456. doi: 10.3389/fimmu.2024.1468456. eCollection 2024. Front Immunol. 2024. PMID: 39450171 Free PMC article. Review.
-
Predominantly defective CD8+ T cell immunity to SARS-CoV-2 mRNA vaccination in lung transplant recipients.J Transl Med. 2023 Jun 8;21(1):374. doi: 10.1186/s12967-023-04234-z. J Transl Med. 2023. PMID: 37291575 Free PMC article.
-
Longitudinal Immunoprofiling of the CD8+ T-Cell Response in SARS-CoV-2 mRNA Vaccinees and COVID-19 Patients.Vaccines (Basel). 2025 May 22;13(6):551. doi: 10.3390/vaccines13060551. Vaccines (Basel). 2025. PMID: 40573882 Free PMC article.
References
-
- Prevention CfDCa . Stay up to date with COVID-19 vaccines including boosters (2022). Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/stay-up-to-date.html#... (Accessed September 29).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

