Heterologous prime-boost cellular vaccination induces potent antitumor immunity against triple negative breast cancer
- PMID: 36860852
- PMCID: PMC9968850
- DOI: 10.3389/fimmu.2023.1098344
Heterologous prime-boost cellular vaccination induces potent antitumor immunity against triple negative breast cancer
Abstract
Introduction: Triple negative breast cancer (TNBC) is the most aggressive and hard-to-treat subtype of breast cancer, affecting 10-20% of all women diagnosed with breast cancer. Surgery, chemotherapy and hormone/Her2 targeted therapies are the cornerstones of treatment for breast cancer, but women with TNBC do not benefit from these treatments. Although the prognosis is dismal, immunotherapies hold significant promise in TNBC, even in wide spread disease because TNBC is infiltrated with more immune cells. This preclinical study is proposing to optimize an oncolytic virus-infected cell vaccine (ICV) based on a prime-boost vaccination strategy to address this unmet clinical need.
Methods: We used various classes of immunomodulators to improve the immunogenicity of whole tumor cells in the prime vaccine, followed by their infection with oncolytic Vesicular Stomatitis Virus (VSVd51) to deliver the boost vaccine. For in vivo studies, we compared the efficacy of a homologous prime-boost vaccination regimen to a heterologous strategy by treating 4T1 tumor bearing BALB/c mice and further by conducting re-challenge studies to evaluate immune memory responses in surviving mice. Due to the aggressive nature of 4T1 tumor spread (akin to stage IV TNBC in human patients), we also compared early surgical resection of primary tumors versus later surgical resection combined with vaccination.
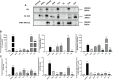
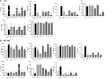
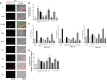
Results: In vitro results demonstrated that immunogenic cell death (ICD) markers and pro-inflammatory cytokines were released at the highest levels following treatment of mouse 4T1 TNBC cells with oxaliplatin chemotherapy and influenza vaccine. These ICD inducers also contributed towards higher dendritic cell recruitment and activation. With the top ICD inducers at hand, we observed that treatment of TNBC-bearing mice with the influenza virus-modified prime vaccine followed by VSVd51 infected boost vaccine resulted in the best survival. Furthermore, higher frequencies of both effector and central memory T cells along with a complete absence of recurrent tumors were observed in re-challenged mice. Importantly, early surgical resection combined with prime-boost vaccination led to improved overall survival in mice.
Conclusion: Taken together, this novel cancer vaccination strategy following early surgical resection could be a promising therapeutic avenue for TNBC patients.
Keywords: Immunogenic cancer vaccine; immune effector cells; oncolytic virotherapy; triple negative breast cancer; tumor microenvironment.
Copyright © 2023 Niavarani, St-Cyr, Daniel, Lawson, Giguère, Alkayyal and Tai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Therapeutic limitations of oncolytic VSVd51-mediated miR-199a-5p delivery in triple negative breast cancer models.Sci Rep. 2025 May 13;15(1):16634. doi: 10.1038/s41598-025-01584-0. Sci Rep. 2025. PMID: 40360587 Free PMC article.
-
Personalized neoantigen viro-immunotherapy platform for triple-negative breast cancer.J Immunother Cancer. 2023 Aug;11(8):e007336. doi: 10.1136/jitc-2023-007336. J Immunother Cancer. 2023. PMID: 37586771 Free PMC article.
-
Liposomal oncolytic adenovirus as a neoadjuvant therapy for triple-negative breast cancer.Sci Rep. 2025 May 14;15(1):16737. doi: 10.1038/s41598-025-00211-2. Sci Rep. 2025. PMID: 40368934 Free PMC article.
-
Progress and Prospect of Immunotherapy for Triple-Negative Breast Cancer.Front Oncol. 2022 Jun 20;12:919072. doi: 10.3389/fonc.2022.919072. eCollection 2022. Front Oncol. 2022. PMID: 35795050 Free PMC article. Review.
-
Targeting molecular cross-talk between tumor cells and tumor associated macrophage as therapeutic strategy in triple negative breast cancer.Int Immunopharmacol. 2023 Jun;119:110250. doi: 10.1016/j.intimp.2023.110250. Epub 2023 May 8. Int Immunopharmacol. 2023. PMID: 37163922 Review.
Cited by
-
Mannan-Decorated Lipid Calcium Phosphate Nanoparticle Vaccine Increased the Antitumor Immune Response by Modulating the Tumor Microenvironment.J Funct Biomater. 2024 Aug 16;15(8):229. doi: 10.3390/jfb15080229. J Funct Biomater. 2024. PMID: 39194667 Free PMC article. Review.
-
Long Prime-Boost Interval and Heightened Anti-GD2 Antibody Response to Carbohydrate Cancer Vaccine.Vaccines (Basel). 2024 May 28;12(6):587. doi: 10.3390/vaccines12060587. Vaccines (Basel). 2024. PMID: 38932316 Free PMC article.
-
The quest for nanoparticle-powered vaccines in cancer immunotherapy.J Nanobiotechnology. 2024 Feb 14;22(1):61. doi: 10.1186/s12951-024-02311-z. J Nanobiotechnology. 2024. PMID: 38355548 Free PMC article. Review.
-
Promoter Hyper-methylation of ZNF662 Restrains its Tumor Suppressing Function in Triple-Negative Breast Cancer Through Regulating NGF Signaling Axis.Int J Biol Sci. 2025 Jun 12;21(9):4081-4097. doi: 10.7150/ijbs.102940. eCollection 2025. Int J Biol Sci. 2025. PMID: 40612670 Free PMC article.
-
Therapeutic limitations of oncolytic VSVd51-mediated miR-199a-5p delivery in triple negative breast cancer models.Sci Rep. 2025 May 13;15(1):16634. doi: 10.1038/s41598-025-01584-0. Sci Rep. 2025. PMID: 40360587 Free PMC article.
References
-
- Adams S, Gray RJ, Demaria S, Goldstein L, Perez EA, Shulman LN, et al. . Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol Off J Am Soc Clin Oncol (2014) 32(27):2959−66. doi: 10.1200/JCO.2013.55.0491 - DOI - PMC - PubMed
-
- Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, et al. . Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: Results from the FinHER trial. Ann Oncol Off J Eur Soc Med Oncol (2014) 25(8):1544−50. doi: 10.1093/annonc/mdu112 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

