Case Report: Nontuberculous mycobacterial infections in children with complete DiGeorge anomaly
- PMID: 36860874
- PMCID: PMC9969526
- DOI: 10.3389/fimmu.2023.1078976
Case Report: Nontuberculous mycobacterial infections in children with complete DiGeorge anomaly
Abstract
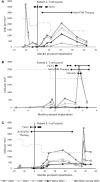
Children with complete DiGeorge anomaly (cDGA) have congenital athymia, resulting in severe T cell immunodeficiency and susceptibility to a broad range of infections. We report the clinical course, immunologic phenotypes, treatment, and outcomes of three cases of disseminated nontuberculous mycobacterial infections (NTM) in patients with cDGA who underwent cultured thymus tissue implantation (CTTI). Two patients were diagnosed with Mycobacterium avium complex (MAC) and one patient with Mycobacterium kansasii. All three patients required protracted therapy with multiple antimycobacterial agents. One patient, who was treated with steroids due to concern for immune reconstitution inflammatory syndrome (IRIS), died due to MAC infection. Two patients have completed therapy and are alive and well. T cell counts and cultured thymus tissue biopsies demonstrated good thymic function and thymopoiesis despite NTM infection. Based on our experience with these three patients, we recommend that providers strongly consider macrolide prophylaxis upon diagnosis of cDGA. We obtain mycobacterial blood cultures when cDGA patients have fevers without a localizing source. In cDGA patients with disseminated NTM, treatment should consist of at least two antimycobacterial medications and be provided in close consultation with an infectious diseases subspecialist. Therapy should be continued until T cell reconstitution is achieved.
Keywords: DiGeorge anomaly; Mycobacterium avium complex; Mycobacterium kansasii; athymia; complete DiGeorge syndrome; nontuberculous mycobacteria; primary immunodeficiency; thymus transplantation.
Copyright © 2023 Hicks, Agada, Yates, Kelly, Tam, Ferdman, Dibernardo, Madden, Moody and Markert.
Conflict of interest statement
Cultured thymus tissue (CTT) is an investigational product implanted into patients under an Investigational New Drug (IND) application with the US Food and Drug Administration. MMa was the “sponsor” of the investigations. MMa developed the technology for CTT. Duke University has licensed the technology to Enzyvant Therapeutics GmbH. MMa and Duke University have received royalties from Enzyvant. Portions of MMa’s and her research team’s salaries are being paid by funding from Enzyvant. If the technology is commercially successful in the future, MMa and Duke University may benefit financially. The salary and other items needed to create CTT are paid at cost by insurance. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Markert ML, Devlin BH, Alexieff MJ, Li J, McCarthy EA, Gupton SE, et al. . Review of 54 patients with complete DiGeorge anomaly enrolled in protocols for thymus transplantation: Outcome of 44 consecutive transplants. Blood (2007) 109(10):4539–47. doi: 10.1182/blood-2006-10-048652 - DOI - PMC - PubMed
-
- Shearer WT, Rosenblatt HM, Gelman RS, Oyomopito R, Plaeger S, Stiehm ER, et al. . Lymphocyte subsets in healthy children from birth through 18 years of age: The pediatric AIDS clinical trials group P1009 study. J Allergy Clin Immunol (2003) 112(5):973–80. doi: 10.1016/j.jaci.2003.07.003 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

