Comprehensive Immunoprofiling of High-Risk Oral Proliferative and Localized Leukoplakia
- PMID: 36860910
- PMCID: PMC9973379
- DOI: 10.1158/2767-9764.CRC-21-0060
Comprehensive Immunoprofiling of High-Risk Oral Proliferative and Localized Leukoplakia
Erratum in
-
Correction: Comprehensive Immunoprofiling of High-risk Oral Proliferative and Localized Leukoplakia.Cancer Res Commun. 2022 May 19;2(5):390. doi: 10.1158/2767-9764.CRC-22-0193. eCollection 2022 May. Cancer Res Commun. 2022. PMID: 36875716 Free PMC article.
Abstract
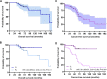
Oral leukoplakia is common and may, in some cases, progress to carcinoma. Proliferative leukoplakia is a progressive, often multifocal subtype with a high rate of malignant transformation compared with the more common localized leukoplakia. We hypothesized that the immune microenvironment and gene expression patterns would be distinct for proliferative leukoplakia compared with localized leukoplakia. We summarize key clinicopathologic features among proliferative leukoplakia and localized leukoplakia and compare cancer-free survival (CFS) between subgroups. We analyze immunologic gene expression profiling in proliferative leukoplakia and localized leukoplakia tissue samples (NanoString PanCancer Immune Oncology Profiling). We integrate immune cell activation and spatial distribution patterns in tissue samples using multiplexed immunofluorescence and digital image capture to further define proliferative leukoplakia and localized leukoplakia. Among N = 58 patients (proliferative leukoplakia, n = 29; localized leukoplakia, n = 29), only the clinical diagnosis of proliferative leukoplakia was associated with significantly decreased CFS (HR, 11.25; P < 0.01; 5-year CFS 46.8% and 83.6% among patients with proliferative leukoplakia and localized leukoplakia, respectively). CD8+ T cells and T regulatory (Treg) were more abundant among proliferative leukoplakia samples (P < 0.01) regardless of degree of epithelial dysplasia, and often colocalized to the dysplasia-stromal interface. Gene set analysis identified granzyme M as the most differentially expressed gene favoring the proliferative leukoplakia subgroup (log2 fold change, 1.93; P adj < 0.001). Programmed death ligand 1 (PD-L1) was comparatively overexpressed among proliferative leukoplakia samples, with higher (>5) PD-L1 scores predicting worse CFS (P adj < 0.01). Proliferative leukoplakia predicts a high rate of malignant transformation within 5 years of diagnosis. A prominent CD8+ T-cell and Treg signature along with relative PD-L1 overexpression compared with localized leukoplakia provides strong rationale for PD-1/PD-L1 axis blockade using preventative immunotherapy.
Significance: This is the first in-depth profiling effort to immunologically characterize high-risk proliferative leukoplakia as compared with the more common localized leukoplakia. We observed a notable cytotoxic T-cell and Treg signature with relative overexpression of PD-L1 in high-risk proliferative leukoplakia providing a strong preclinical rationale for investigating PD-1/PD-L1 axis blockade in this disease as preventative immunotherapy.
© 2021 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
G.J. Hanna reports grants from DFCI Medical Oncology Program during the conduct of the study, as well as other (sponsored research) from BMS, personal fees and other (sponsored research) from Exicure, grants from Gateway for Cancer Research, other (sponsored research) from Kite, other (sponsored research) from NantKwest/Altor Bioscience, personal fees and other (sponsored research) from Regeneron, personal fees and other (sponsored research) from Sanofi Genzyme, personal fees and other (sponsored research) from Bicara, personal fees from Maverick, and personal fees from Merck outside the submitted work. R.I. Haddad reports grants and personal fees from Merck, BMS, AstraZeneca, Genentech, Pfizer, Eisai, Bayer, GSK outside the submitted work. R. Uppaluri reports personal fees from Merck outside the submitted work. S.J. Rodig reports grants from Bristol Myers Squibb, grants from KITE/Gilead, grants from Merck, grants from Affimed Inc., and other from Immunitas Inc outside the submitted work. S.-B. Woo reports grants from Bristol Myers Squibb outside the submitted work. F.S. Hodi reports personal fees from Merck, Novartis, Aduro, Pionyr, Checkpoint, Surface, Compass, Torque, Rheos, Bicara, Genentech, Takeda, Eisai, Iovance, Bioentre, Gossamer, Novartis, 7Hills, Amgen, and Immunocore outside the submitted work, as well as patents for the following: MICA Related Disorders (licensed to institution per institutional policies); Tumor antigens and uses thereof (issued to institution per institutional policies); Angiopoiten-2 Biomarkers Predictive of Anti-immune checkpoint response (pending to institution per institutional policies); Compositions and Methods for Identification, Assessment, Prevention, and Treatment of Melanoma using PD-L1 Isoforms (pending to institution per institutional policies; Therapeutic peptides (issued to institution per institutional policies); Vaccine compositions and methods for restoring NKG2D pathway function against cancers (licensed to institution per institutional policies); Antibodies that bind to MHC class I polypeptide-related sequence A (licensed to to institution per institutional policies); and ANTI-GALECTIN ANTIBODY BIOMARKERS PREDICTIVE OF ANTI-IMMUNE CHECKPOINT AND ANTI-ANGIOGENESIS RESPONSES (pending to institution per institutional policies.)
Figures




References
-
- Villa A, Woo SB. Leukoplakia-a diagnostic and management algorithm. J Oral Maxillofac Surg 2016;75:723–34. - PubMed
-
- Warnakulasuriya S, Johnson NW, van der Waal I. Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med 2007;36:575–80. - PubMed
-
- Mello FW, Miguel AFP, Dutra KL, Porporatti AL, Warnakulasuriya S, Guerra ENS, et al. . Prevalence of oral potentially malignant disorders: a systematic review and meta-analysis. J Oral Pathol Med 2018;47:633–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

