Dietary Fish Meal Level and a Package of Choline, β-Glucan, and Nucleotides Modulate Gut Function, Microbiota, and Health in Atlantic Salmon (Salmo salar, L.)
- PMID: 36860972
- PMCID: PMC9973201
- DOI: 10.1155/2023/5422035
Dietary Fish Meal Level and a Package of Choline, β-Glucan, and Nucleotides Modulate Gut Function, Microbiota, and Health in Atlantic Salmon (Salmo salar, L.)
Abstract
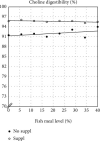
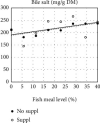
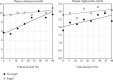
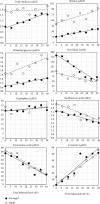
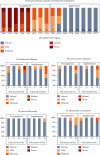
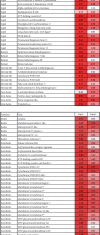
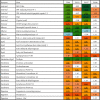
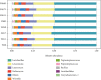
Steatosis and inflammation have been common gut symptoms in Atlantic salmon fed plant rich diets. Choline has recently been identified as essential for salmon in seawater, and β-glucan and nucleotides are frequently used to prevent inflammation. The study is aimed at documenting whether increased fishmeal (FM) levels (8 levels from 0 to 40%) and supplementation (Suppl) with a mixture of choline (3.0 g/kg), β-glucan (0.5 g/kg), and nucleotides (0.5 g/kg) might reduce the symptoms. Salmon (186 g) were fed for 62 days in 16 saltwater tanks before samples were taken from 12 fish per tank for observation of biochemical, molecular, metabolome, and microbiome indicators of function and health. Steatosis but no inflammation was observed. Lipid digestibility increased and steatosis decreased with increasing FM levels and supplementation, seemingly related to choline level. Blood metabolites confirmed this picture. Genes in intestinal tissue affected by FM levels are mainly involved in metabolic and structural functions. Only a few are immune genes. The supplement reduced these FM effects. In gut digesta, increasing FM levels increased microbial richness and diversity, and changed the composition, but only for unsupplemented diets. An average choline requirement of 3.5 g/kg was indicated for Atlantic salmon at the present life stage and under the present condition.
Copyright © 2023 Åshild Krogdahl et al.
Conflict of interest statement
None of the authors of this manuscript has any conflict of interest regarding the work presented in this manuscript.
Figures










References
-
- Penn M. H. Annual Report on Fish Health . Norwegian Institute of Veterinary Medicine; 2011. Lipid malabsorption in Atlantic Salmon – the recurring problem of floating feces.
-
- NRC. NRC Nutrient Requirement of Fish and Shrimp . National Academic Press; 2011. Vitamins; pp. 186–220.
-
- Krogdahl A., Hansen A. K. G., Kortner T. M., et al. Choline and phosphatidylcholine, but not methionine, cysteine, taurine and taurocholate, eliminate excessive gut mucosal lipid accumulation in Atlantic salmon (Salmo salar L) Aquaculture . 2020;528:p. 735552. doi: 10.1016/j.aquaculture.2020.735552. - DOI
LinkOut - more resources
Full Text Sources
