Leaf hydroalcoholic extract and oleoresin from Copaifera multijuga control Toxoplasma gondii infection in human trophoblast cells and placental explants from third-trimester pregnancy
- PMID: 36860986
- PMCID: PMC9970041
- DOI: 10.3389/fcimb.2023.1113896
Leaf hydroalcoholic extract and oleoresin from Copaifera multijuga control Toxoplasma gondii infection in human trophoblast cells and placental explants from third-trimester pregnancy
Abstract
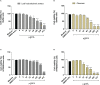
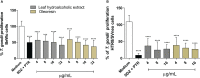
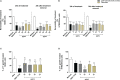
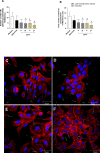
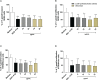
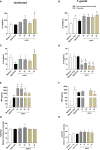
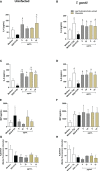
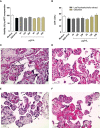
The conventional treatment of congenital toxoplasmosis is mainly based on the combination of sulfadiazine and pyrimethamine. However, therapy with these drugs is associated with severe side effects and resistance, requiring the study of new therapeutic strategies. There are currently many studies with natural products, including Copaifera oleoresin, showing actions against some pathogens, as Trypanosoma cruzi and Leishmania. In the present study, we investigated the effects of the leaf hydroalcoholic extract and oleoresin from Copaifera multijuga against Toxoplasma gondii in human villous (BeWo) and extravillous (HTR8/SVneo) trophoblast cells, as well as in human villous explants from third-trimester pregnancy. For this purpose, both cells and villous explants were infected or not with T. gondii, treated with hydroalcoholic extract or oleoresin from C. multijuga and analyzed for toxicity, parasite proliferation, cytokine and ROS production. In parallel, both cells were infected by tachyzoites pretreated with hydroalcoholic extract or oleoresin, and adhesion, invasion and replication of the parasite were observed. Our results showed that the extract and oleoresin did not trigger toxicity in small concentrations and were able to reduce the T. gondii intracellular proliferation in cells previously infected. Also, the hydroalcoholic extract and oleoresin demonstrated an irreversible antiparasitic action in BeWo and HTR8/SVneo cells. Next, adhesion, invasion and replication of T. gondii were dampened when BeWo or HTR8/SVneo cells were infected with pretreated tachyzoites. Finally, infected and treated BeWo cells upregulated IL-6 and downmodulated IL-8, while HTR8/SVneo cells did not change significantly these cytokines when infected and treated. Finally, both the extract and oleoresin reduced the T. gondii proliferation in human explants, and no significant changes were observed in relation to cytokine production. Thus, compounds from C. multijuga presented different antiparasitic activities that were dependent on the experimental model, being the direct action on tachyzoites a common mechanism operating in both cells and villi. Considering all these parameters, the hydroalcoholic extract and oleoresin from C. multijuga can be a target for the establishment of new therapeutic strategy for congenital toxoplasmosis.
Keywords: Copaifera multijuga; Toxoplasma gondii; congenital toxoplasmosis (CT); hydroalcoholic extract; oleoresin; trophoblast.
Copyright © 2023 Martínez, Teixeira, de Souza, Rosini, Júnior, Melo, Blandón, Gomes, Ambrósio, Veneziani, Bastos, Martins, Ferro and Barbosa.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Ahmadpour E., Zargami E., Mahami-Oskouei M., Spotin A., Shahbazi A., Kafil H. S., et al. . (2019). Diagnosis of Toxoplasma gondii infection in pregnant women using automated chemiluminescence and quantitative real time PCR. Asian Pac. J. Trop. Med. 12 (1), 26–31. doi: 10.4103/1995-7645.250341 - DOI
-
- Arruda C., Mejía J. A. A., Ribeiro V. P., Borges C. H. G., Martins C. H. G., Veneziani R. C. S., et al. . (2019). Occurrence, chemical composition, biological activities and analytical methods on Copaifera genus - a review. Biomed. Pharmacother. 109, 1–20. doi: 10.1016/j.biopha.2018.10.030 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

