At-home noninvasive ventilation initiation with telemonitoring in amyotrophic lateral sclerosis patients: a retrospective study
- PMID: 36861058
- PMCID: PMC9969309
- DOI: 10.1183/23120541.00438-2022
At-home noninvasive ventilation initiation with telemonitoring in amyotrophic lateral sclerosis patients: a retrospective study
Abstract
Background: Noninvasive ventilation (NIV) improves survival and quality of life in amyotrophic lateral sclerosis (ALS) patients. NIV initiation is mostly conducted at hospital, but a recurrent lack of hospital beds led to the necessity of exploring an at-home initiation process. Here, we report data from our NIV initiation cohort of ALS patients. Could our at-home NIV initiation process with telemonitoring in ALS patients be an efficient solution for adherence and nocturnal hypoxaemia correction?
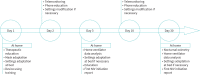
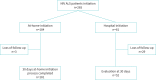
Methods: We performed a retrospective analysis of data collected from 265 ALS patients treated at the Bordeaux ALS Centre for whom NIV initiation was carried out between September 2017 and June 2021, with two modalities: at-home initiation or in-hospital initiation. The primary outcome was adherence to NIV at 30 days. The secondary outcome was at-home NIV initiation process efficiency of nocturnal hypoxaemia correction.
Results: At 30 days, NIV adherence (mean >4 h·day-1) was 66% of the total population, 70% of the at-home NIV initiation subgroup and 52% of the in-hospital NIV initiation subgroup. Nocturnal hypoxaemia correction was observed in 79% of adherent patients in the at-home NIV initiation subgroup. Mean delay of NIV prescription and at-home NIV initiation was 8.7 days (+/-6.5) versus 29.5 days in hospital.
Conclusion: Our study shows that our at-home NIV initiation process in ALS patients is a good option to provide rapid access to NIV with good adherence and efficiency. Further literature on the benefits of at-home NIV initiation is welcomed, especially to evaluate long-term efficiency and global cost analysis.
Copyright ©The authors 2023.
Conflict of interest statement
Conflict of interest: T. Réginault reports support for the present manuscript from the Bordeaux University Foundation; and travel support from Vivisol and personal fees from Zéphyr Paramed, outside the submitted work. B. Bouteleux reports personal fees from Zéphyr Paramed outside the submitted work. P. Wibart reports support for the present manuscript from the Bordeaux University Foundation, and personal fees from Zéphyr Paramed outside the submitted work. L. Grassion received grants or contracts from AADAIRC, outside the submitted work; payment of honoraria for lectures, presentations, speakers' bureaus, manuscript writing or educational events from SOS Oxygene, ASTEN Santé, and ALMS, outside the submitted work; support for attending meetings and/or travel from SOS Oxygen, VIVISOL and ASTEN Santé, outside the submitted work; and participation on a data safety monitoring board or advisory board for VIVISOL, outside the submitted work. All other authors have nothing to disclose.
Figures
References
-
- Perez T. [Amyotrophic lateral sclerosis (ALS): evaluation of respiratory function]. Rev Neurol (Paris) 2006; 162: Spec. No. 2, 4S188–4S194. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
