Comprehensive Metabolic Tracing Reveals the Origin and Catabolism of Cysteine in Mammalian Tissues and Tumors
- PMID: 36862034
- PMCID: PMC10152234
- DOI: 10.1158/0008-5472.CAN-22-3000
Comprehensive Metabolic Tracing Reveals the Origin and Catabolism of Cysteine in Mammalian Tissues and Tumors
Erratum in
-
Correction: Comprehensive Metabolic Tracing Reveals the Origin and Catabolism of Cysteine in Mammalian Tissues and Tumors.Cancer Res. 2024 Apr 15;84(8):1372. doi: 10.1158/0008-5472.CAN-24-0459. Cancer Res. 2024. PMID: 38616660 Free PMC article. No abstract available.
Abstract
Cysteine plays critical roles in cellular biosynthesis, enzyme catalysis, and redox metabolism. The intracellular cysteine pool can be sustained by cystine uptake or de novo synthesis from serine and homocysteine. Demand for cysteine is increased during tumorigenesis for generating glutathione to deal with oxidative stress. While cultured cells have been shown to be highly dependent on exogenous cystine for proliferation and survival, how diverse tissues obtain and use cysteine in vivo has not been characterized. We comprehensively interrogated cysteine metabolism in normal murine tissues and cancers that arise from them using stable isotope 13C1-serine and 13C6-cystine tracing. De novo cysteine synthesis was highest in normal liver and pancreas and absent in lung tissue, while cysteine synthesis was either inactive or downregulated during tumorigenesis. In contrast, cystine uptake and metabolism to downstream metabolites was a universal feature of normal tissues and tumors. However, differences in glutathione labeling from cysteine were evident across tumor types. Thus, cystine is a major contributor to the cysteine pool in tumors, and glutathione metabolism is differentially active across tumor types.
Significance: Stable isotope 13C1-serine and 13C6-cystine tracing characterizes cysteine metabolism in normal murine tissues and its rewiring in tumors using genetically engineered mouse models of liver, pancreas, and lung cancers.
©2023 The Authors; Published by the American Association for Cancer Research.
Figures


![Figure 2. Contribution of de novo cysteine synthesis to the cysteine pool varies across healthy mouse tissues. A, Schematic depicting 1-[13C1]-serine infusion and its metabolism via the transsulfuration and glutathione synthesis pathways. B–G, Healthy C57BL/6J mice were infused with 1-[13C1]-serine, followed by analysis of the fraction labeling in serine (B), glycine (C), cystathionine (D), glutathione (E), cysteine (F), and γ-glutamylcysteine (G). For B–G, data are presented as mean ± SD and N = 10 mice (5 male, 5 female). N.D., not detected. H, Fractional contribution of serine to intracellular cysteine synthesis in each tissue from B–G. Cysteine labeling was normalized to the fraction labeling of serine in each tissue. I, Immunoblots of CBS and CSE for each tissue. HSP90 was used for the loading control. αKB, α-ketobutyrate; Cth, cystathionine; Cys, cysteine; Gly, glycine; Glut, glutamate; GSH, glutathione; γ-Glu-Cys, γ-glutamylcysteine; Ser, serine. (A, Created with BioRender.com.)](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/dacd/10152234/7119581561fc/1426fig2.gif)
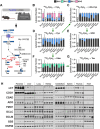
![Figure 4. Tumorigenesis of liver and pancreas induces downregulation of de novo cysteine synthesis. A, Schematic for the generation of Myc; p53−/− HCC and KrasG12D; p53+/− PDAC GEMM tumors. B, Analysis of the fraction labeling in serine, glycine, cystathionine, glutathione, cysteine, and γ-glutamylcysteine in liver tissues (N = 8) compared with HCC tumors (N = 8) and their matched serum normal (N = 8) and HCC (N = 5) following infusion with 1-[13C1]-serine. C, Analysis of the fraction labeling in serine, glycine, cystathionine, glutathione, cysteine, and γ-glutamylcysteine in pancreas tissues (N = 5) compared with PDAC tumors (N = 5) and their matched serum from normal (N = 5) and PDAC (N = 5) following infusion with 1-[13C1]-serine. D, Fractional contribution of serine to intracellular cysteine synthesis in HCC and PDAC. Cysteine labeling was normalized to the fraction labeling of serine in each tissue. One healthy pancreas sample was excluded because of a division error. For B–D, data are presented as mean ± SD. N.D., not detected. E, Immunoblots of CBS and CSE for each tissue. HSP90 was used for the loading control. *, P < 0.05; **, P < 0.01. Cth, cystathionine; Cys, cysteine; Gly, glycine; GSH, glutathione; γ-Glu-Cys, γ-glutamylcysteine; Ser, serine. (A, Created with BioRender.com.)](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/dacd/10152234/f09e8d89c277/1426fig4.gif)
![Figure 5. De novo cysteine synthesis does not contribute to the cysteine pool of lung tumors. A, Schematic for the generation of KrasG12D; p53−/− and KrasG12D; p53−/−; Nrf2D29H LUAD, and Rb1−/−; p53−/−; MycT58A/+ or Rb1−/−; p53−/−; MycT58A/T58A SCLC GEMM tumors. B, Analysis of the fraction labeling in serine, glycine, cystathionine, glutathione, cysteine, and γ-glutamylcysteine in normal lung tissues (N = 8) compared with Nrf2WT LUAD and (N = 10), Nrf2D29H LUAD tumors (N = 10) and their matched serum from normal (N = 8), Nrf2WT (N = 5), and Nrf2D29H (N = 5) following infusion with 1-[13C1]-serine. C, Analysis of the fraction labeling in serine, glycine, cystathionine, glutathione, cysteine, and γ-glutamylcysteine in normal lung tissues (N = 8) compared with SCLC tumors (N = 9), and their matched serum normal (N = 8) and SCLC (N = 9) following infusion with 1-[13C1]-serine. The control lung samples in C are the same as in B. D, Fractional contribution of serine to intracellular cysteine synthesis in LUAD and SCLC. Cysteine labeling was normalized to the fraction labeling of serine in each tissue. For B–D, data are presented as mean ± SD. N.D., not detected. E, Immunoblots of CBS and CSE for each tissue. HSP90 was used for the loading control. *, P < 0.05; ****, P < 0.0001. Cth, cystathionine; Cys, cysteine; Gly, glycine; GSH, glutathione; γ-Glu-Cys, γ-glutamylcysteine; Ser, serine. (A, Created with BioRender.com.)](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/dacd/10152234/2db5a4c2b140/1426fig5.gif)

References
-
- Ward NP, DeNicola GM. Sulfur metabolism and its contribution to malignancy. Int Rev Cell Mol Biol 2019;347:39–103. - PubMed
-
- Winterbourn CC, Hampton MB. Thiol chemistry and specificity in redox signaling. Free Radical Biol Med 2008;45:549–61. - PubMed
-
- Anderson ME. Glutathione: an overview of biosynthesis and modulation. Chem Biol Interact 1998;111–112:1–14. - PubMed
-
- Lu SC. Regulation of hepatic glutathione synthesis: current concepts and controversies. FASEB J 1999;13:1169–83. - PubMed
-
- Harris IS, DeNicola GM. The complex interplay between antioxidants and ROS in cancer. Trends Cell Biol 2020;30:440–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

