Dynamic tilting in perovskites
- PMID: 36862041
- PMCID: PMC9979940
- DOI: 10.1107/S2053273322011949
Dynamic tilting in perovskites
Abstract
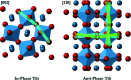
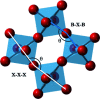
A new computational analysis of tilt behaviour in perovskites is presented. This includes the development of a computational program - PALAMEDES - to extract tilt angles and the tilt phase from molecular dynamics simulations. The results are used to generate simulated selected-area electron and neutron diffraction patterns which are compared with experimental patterns for CaTiO3. The simulations not only reproduced all symmetrically allowed superlattice reflections associated with tilt but also showed local correlations that give rise to symmetrically forbidden reflections and the kinematic origin of diffuse scattering.
Keywords: diffraction; molecular dynamics; perovskites; superlattice; tilt.
open access.
Figures



References
-
- Amat, A., Mosconi, E., Ronca, E., Quarti, C., Umari, P., Nazeeruddin, M. K., Grätzel, M. & De Angelis, F. (2014). Nano Lett. 14, 3608–3616. - PubMed
-
- Beanland, R. (2011). Acta Cryst. A67, 191–199. - PubMed
-
- Beran, A., Libowitzky, E. & Armbruster, T. (1996). Can. Miner. 34, 803–809.
-
- Cockayne, E. & Burton, B. P. (2000). Phys. Rev. B, 62, 3735–3743.
Grants and funding
LinkOut - more resources
Full Text Sources

