Outcomes of hepatocellular carcinoma by etiology with first-line atezolizumab and bevacizumab: a real-world analysis
- PMID: 36862158
- PMCID: PMC11044531
- DOI: 10.1007/s00432-023-04590-9
Outcomes of hepatocellular carcinoma by etiology with first-line atezolizumab and bevacizumab: a real-world analysis
Abstract
Purpose: Hepatocellular carcinoma (HCC) is a common and deadly form of liver cancer. Combination atezolizumab and bevacizumab has improved the outcomes for patients with advanced disease. We sought to determine the impact of etiology on outcomes of patients treated with atezolizumab and bevacizumab.
Methods: This study used a real-world database. The primary outcome was overall survival (OS) by etiology of HCC; the secondary outcome was real-world time to treatment discontinuation (rwTTD). Time-to-event analyses was performed by the Kaplan-Meier method; the log-rank test to assess for differences by etiology from date of first receipt of atezolizumab and bevacizumab. The Cox proportional hazards model was used to calculate hazard ratios.
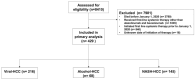
Results: In total, 429 patients were included (n = 216 Viral-HCC; n = 68 Alcohol-HCC; n = 145, NASH-HCC). The median overall survival for the entire cohort was 9.4 months (95% CI 7.1-10.9). Compared with Viral-HCC, the hazard ratio (HR) of death was 1.11 (95% CI 0.74-1.68, p = 0.62) for Alcohol-HCC and was 1.34 (95% CI 0.96-1.86, p = 0.08) for NASH-HCC. The median rwTTD for the entire cohort was 5.7 months (95% CI 5.0-7.0 months). The HR of rwTTD was 1.24 (95% CI 0.86-1.77, p = 0.25) for Alcohol-HCC and was 1.31 (95% CI 0.98-1.75, p = 0.06) in reference to TTD with Viral-HCC.
Conclusions: In this real-world cohort of patients with HCC receiving first-line atezolizumab and bevacizumab, we did not identify an association between etiology and OS or rwTTD. This suggests that the efficacy of atezolizumab and bevacizumab may be similar across HCC etiologies. Further prospective studies are needed to confirm these findings.
Keywords: Hepatocellular carcinoma; Immunotherapy; NASH; Outcomes.
© 2023. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
The authors have not disclosed any competing interests.
Figures

References
-
- Abou-Alfa GK, Lau G, Kudo M et al (2022) Tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. NEJM Evid. 10.1056/EVIDoa2100070 - PubMed
-
- Anstee QM, Reeves HL, Kotsiliti E et al (2019) From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol 16:411–428. 10.1038/s41575-019-0145-7 - PubMed
-
- Birnbaum B, Nussbaum N, Seidl-Rathkopf K et al (2020) Model-assisted cohort selection with bias analysis for generating large-scale cohorts from the EHR for oncology research
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

