PPM1K-regulated impaired catabolism of branched-chain amino acids orchestrates polycystic ovary syndrome
- PMID: 36863088
- PMCID: PMC9986518
- DOI: 10.1016/j.ebiom.2023.104492
PPM1K-regulated impaired catabolism of branched-chain amino acids orchestrates polycystic ovary syndrome
Abstract
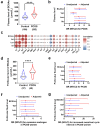
Background: Polycystic ovary syndrome (PCOS) is one of the most common diseases with the coexistence of reproductive malfunction and metabolic disorders. Previous studies have found increased branched chain amino acid (BCAA) levels in women with PCOS. However, it remains unclear whether BCAA metabolism is causally associated with the risk of PCOS.
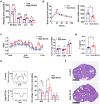
Methods: The changes of BCAA levels in the plasma and follicular fluids of PCOS women were detected. Mendelian randomization (MR) approaches were used to explore the potential causal association between BCAA levels and the risk of PCOS. The function of the gene coding the protein phosphatase Mg2+/Mn2+-dependent 1K (PPM1K) was further explored by using Ppm1k-deficient mouse model and PPM1K down-regulated human ovarian granulosa cells.
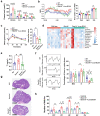
Findings: BCAA levels were significantly elevated in both plasma and follicular fluids of PCOS women. Based on MR, a potential direct, causal role for BCAA metabolism was revealed in the pathogenesis of PCOS, and PPM1K was detected as a vital driver. Ppm1k-deficient female mice had increased BCAA levels and exhibited PCOS-like traits, including hyperandrogenemia and abnormal follicle development. A reduction in dietary BCAA intake significantly improved the endocrine and ovarian dysfunction of Ppm1k-/- female mice. Knockdown of PPM1K promoted the conversion of glycolysis to pentose phosphate pathway and inhibited mitochondrial oxidative phosphorylation in human granulosa cells.
Interpretation: Ppm1k deficiency-impaired BCAA catabolism causes the occurrence and development of PCOS. PPM1K suppression disturbed energy metabolism homeostasis in the follicular microenvironment, which provided an underlying mechanism of abnormal follicle development.
Funding: This study was supported by the National Key Research and Development Program of China (2021YFC2700402, 2019YFA0802503), the National Natural Science Foundation of China (81871139, 82001503, 92057107), the CAMS Innovation Fund for Medical Sciences (2019-I2M-5-001), Key Clinical Projects of Peking University Third Hospital (BYSY2022043), the China Postdoctoral Science Foundation (2021T140600), and the Collaborative Innovation Program of Shanghai Municipal Health Commission (2020CXJQ01).
Keywords: BCAA Catabolism; Mendelian randomization; PCOS; PPM1K.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare that they have no competing interests.
Figures




References
-
- Skiba M.A., Islam R.M., Bell R.J., Davis S.R. Understanding variation in prevalence estimates of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2018;24(6):694–709. - PubMed
-
- Escobar-Morreale H.F. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14(5):270–284. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

