Engineering pancreatic islets with a novel form of thrombomodulin protein to overcome early graft loss triggered by instant blood-mediated inflammatory reaction
- PMID: 36863480
- PMCID: PMC10318623
- DOI: 10.1016/j.ajt.2023.02.021
Engineering pancreatic islets with a novel form of thrombomodulin protein to overcome early graft loss triggered by instant blood-mediated inflammatory reaction
Abstract
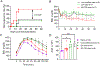
The instant blood-mediated inflammatory reaction (IBMIR) is initiated by innate immune responses that cause substantial islet loss after intraportal transplantation. Thrombomodulin (TM) is a multifaceted innate immune modulator. In this study, we report the generation of a chimeric form of thrombomodulin with streptavidin (SA-TM) for transient display on the surface of islets modified with biotin to mitigate IBMIR. SA-TM protein expressed in insect cells showed the expected structural and functional features. SA-TM converted protein C into activated protein C, blocked phagocytosis of xenogeneic cells by mouse macrophages and inhibited neutrophil activation. SA-TM was effectively displayed on the surface of biotinylated islets without a negative effect on their viability or function. Islets engineered with SA-TM showed improved engraftment and established euglycemia in 83% of diabetic recipients when compared with 29% of recipients transplanted with SA-engineered islets as control in a syngeneic minimal mass intraportal transplantation model. Enhanced engraftment and function of SA-TM-engineered islets were associated with the inhibition of intragraft proinflammatory innate cellular and soluble mediators of IBMIR, such as macrophages, neutrophils, high-mobility group box 1, tissue factor, macrophage chemoattractant protein-1, interleukin-1β, interleukin-6, tumor necrosis factor-α, interferon-γ. Transient display of SA-TM protein on the islet surface to modulate innate immune responses causing islet graft destruction has clinical potential for autologous and allogeneic islet transplantation.
Keywords: immunomodulation; instant blood-mediated inflammatory reaction; intraportal transplantation; islet transplantation; thrombomodulin; thrombomodulin chimeric with streptavidin innate immunity.
Copyright © 2023 American Society of Transplantation & American Society of Transplant Surgeons. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
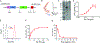





References
-
- Naziruddin B, Iwahashi S, Kanak MA, Takita M, Itoh T, Levy MF. Evidence for instant blood-mediated inflammatory reaction in clinical autologous islet transplantation. Am J Transplant 2014;14(2):428–437. - PubMed
-
- Bennet W, Sundberg B, Groth CG, Brendel MD, Brandhorst D, Brandhorst H et al. Incompatibility between human blood and isolated islets of Langerhans: a finding with implications for clinical intraportal islet transplantation? Diabetes 1999;48(10):1907–1914. - PubMed
-
- Moberg L, Johansson H, Lukinius A, Berne C, Foss A, Kallen R et al. Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet 2002;360(9350):2039–2045. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

