Type I interferon pathway assays in studies of rheumatic and musculoskeletal diseases: a systematic literature review informing EULAR points to consider
- PMID: 36863752
- PMCID: PMC9990675
- DOI: 10.1136/rmdopen-2022-002876
Type I interferon pathway assays in studies of rheumatic and musculoskeletal diseases: a systematic literature review informing EULAR points to consider
Abstract
Objectives: To systematically review the literature for assay methods that aim to evaluate type I interferon (IFN-I) pathway activation and to harmonise-related terminology.
Methods: Three databases were searched for reports of IFN-I and rheumatic musculoskeletal diseases. Information about the performance metrics of assays measuring IFN-I and measures of truth were extracted and summarised. A EULAR task force panel assessed feasibility and developed consensus terminology.
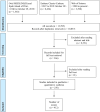
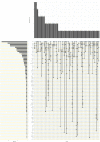
Results: Of 10 037 abstracts, 276 fulfilled eligibility criteria for data extraction. Some reported more than one technique to measure IFN-I pathway activation. Hence, 276 papers generated data on 412 methods. IFN-I pathway activation was measured using: qPCR (n=121), immunoassays (n=101), microarray (n=69), reporter cell assay (n=38), DNA methylation (n=14), flow cytometry (n=14), cytopathic effect assay (n=11), RNA sequencing (n=9), plaque reduction assay (n=8), Nanostring (n=5), bisulphite sequencing (n=3). Principles of each assay are summarised for content validity. Concurrent validity (correlation with other IFN assays) was presented for n=150/412 assays. Reliability data were variable and provided for 13 assays. Gene expression and immunoassays were considered most feasible. Consensus terminology to define different aspects of IFN-I research and practice was produced.
Conclusions: Diverse methods have been reported as IFN-I assays and these differ in what elements or aspects of IFN-I pathway activation they measure and how. No 'gold standard' represents the entirety of the IFN pathway, some may not be specific for IFN-I. Data on reliability or comparing assays were limited, and feasibility is a challenge for many assays. Consensus terminology should improve consistency of reporting.
Keywords: Arthritis, Rheumatoid; Cytokines; Inflammation; Lupus Erythematosus, Systemic.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: MKC has received consulting fees from AstraZeneca, Bristol Meyers Squibb, Lilly and Shannon Pharmaceuticals, as well as grant/research support from Gilead. LR has received consulting fees from AstraZeneca. EV served in the speakers’ bureau of GSK, received consulting fees from AURINIA, SANDOZ, GSK, AstraZeneca, Roche, and Modus, as well as grant/research support from AstraZeneca. PGC has received consultancies or speaker fees from AbbVie, Amgen, AstraZeneca, BMS, Eli Lilly, Galapagos, GSK, Merck, Pfizer, Novartis and UCB. The rest of the authors have no conflict of interest to declare.
Figures




References
-
- Lamot L, Niemietz I, Brown KL. Methods for type I interferon detection and their relevance for clinical utility and improved understanding of rheumatic diseases. Clin Exp Rheumatol 2019;37:1077–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
