Assessing non-adjunctive CGM safety at home and in new markets (ANSHIN)
- PMID: 36864014
- PMCID: PMC10164430
- DOI: 10.1002/edm2.414
Assessing non-adjunctive CGM safety at home and in new markets (ANSHIN)
Abstract
Introduction: Continuous glucose monitoring (CGM) can guide treatment for people with type 1 (T1D) and type 2 diabetes (T2D). The ANSHIN study assessed the impact of non-adjunctive CGM use in adults with diabetes using intensive insulin therapy (IIT).
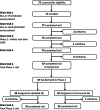
Materials and methods: This single-arm, prospective, interventional study enrolled adults with T1D or T2D who had not used CGM in the prior 6 months. Participants wore blinded CGMs (Dexcom G6) during a 20-day run-in phase, with treatment based on fingerstick glucose values, followed by a 16-week intervention phase and then a randomized 12-week extension phase with treatment based on CGM values. The primary outcome was change in HbA1c. Secondary outcomes were CGM metrics. Safety endpoints were the number of severe hypoglycaemic (SH) and diabetic ketoacidosis (DKA) events.
Results: Of the 77 adults enrolled, 63 completed the study. Those enrolled had mean (SD) baseline HbA1c of 9.8% (1.9%), 36% had T1D, and 44% were ≥65 years old. Mean HbA1c decreased by 1.3, 1.0 and 1.0 percentage points for participants with T1D, T2D or age ≥65, respectively (p < .001 for each). CGM-based metrics including time in range also improved significantly. SH events decreased from the run-in period (67.3 per 100 person-years) to the intervention period (17.0 per 100 person-years). Three DKA events unrelated to CGM use occurred during the total intervention period.
Conclusions: Non-adjunctive use of the Dexcom G6 CGM system improved glycaemic control and was safe for adults using IIT.
Keywords: blood glucose self-monitoring; glycaemic control; glycated haemoglobin.
© 2023 The Authors. Endocrinology, Diabetes & Metabolism published by John Wiley & Sons Ltd.
Figures
References
-
- Karter AJ, Ackerson LM, Darbinian JA, et al. Self‐monitoring of blood glucose levels and glycemic control: the northern California Kaiser Permanente Diabetes registry. Am J Med. 2001;111(1):1‐9. - PubMed
-
- Pettus J, Price DA, Edelman SV. How patients with type 1 Diabetes translate continuous glucose monitoring data into Diabetes management decisions. Endocr Pract. 2015;21(6):613‐620. - PubMed
-
- Beck RW, Riddlesworth T, Ruedy K, et al. Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA. 2017;317(4):371‐378. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous