Prognosis after discontinuing renin angiotensin aldosterone system inhibitor for heart failure with restored ejection fraction after acute myocardial infarction
- PMID: 36864119
- PMCID: PMC9981744
- DOI: 10.1038/s41598-023-30700-1
Prognosis after discontinuing renin angiotensin aldosterone system inhibitor for heart failure with restored ejection fraction after acute myocardial infarction
Abstract
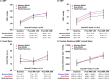
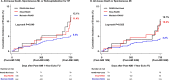
Prognostic effect of discontinuing renin-angiotensin-aldosterone-system-inhibitor (RAASi) for patients with heart failure (HF) after acute myocardial infarction (AMI) whose left ventricular (LV) systolic function was restored during follow-up is unknown. To investigate the outcome after discontinuing RAASi in post-AMI HF patients with restored LV ejection fraction (EF). Of 13,104 consecutive patients from the nationwide, multicenter, and prospective Korea Acute Myocardial Infarction-National Institutes of Health (KAMIR-NIH) registry, HF patients with baseline LVEF < 50% that was restored to ≥ 50% at 12-month follow-up were selected. Primary outcome was a composite of all-cause death, spontaneous MI, or rehospitalization for HF at 36-month after index procedure. Of 726 post-AMI HF patients with restored LVEF, 544 maintained RAASi (Maintain-RAASi) beyond 12-month, 108 stopped RAASi (Stop-RAASi), and 74 did not use RAASi (RAASi-Not-Used) at baseline and follow-up. Systemic hemodynamics and cardiac workloads were similar among groups at baseline and during follow-up. Stop-RAASi group showed elevated NT-proBNP than Maintain-RAASi group at 36-month. Stop-RAASi group showed significantly higher risk of primary outcome than Maintain-RAASi group (11.4% vs. 5.4%; adjusted hazard ratio [HRadjust] 2.20, 95% confidence interval [CI] 1.09-4.46, P = 0.028), mainly driven by increased risk of all-cause death. The rate of primary outcome was similar between Stop-RAASi and RAASi-Not-Used group (11.4% vs. 12.1%; HRadjust 1.18 [0.47-2.99], P = 0.725). In post-AMI HF patients with restored LV systolic function, RAASi discontinuation was associated with significantly increased risk of all-cause death, MI, or rehospitalization for HF. Maintaining RAASi will be necessary for post-AMI HF patients, even after LVEF is restored.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- McDonagh TA, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

