Plant growth promotion and differential expression of defense genes in chilli pepper against Colletotrichum truncatum induced by Trichoderma asperellum and T. harzianum
- PMID: 36864373
- PMCID: PMC9983198
- DOI: 10.1186/s12866-023-02789-x
Plant growth promotion and differential expression of defense genes in chilli pepper against Colletotrichum truncatum induced by Trichoderma asperellum and T. harzianum
Abstract
Background: Trichoderma asperellum and T. harzianum were assessed in this study as a potential biological control against Colletotrichum truncatum. C. truncatum is a hemibiotrophic fungus that causes anthracnose disease in chilli thereby affecting plant growth and fruit yield. Scanning electron microscope (SEM) technique showed the beneficial interaction between chilli root-Trichoderma spp. inducing the plant growth promotion, mechanical barrier, and defense network under C. truncatum challenged conditions.
Methods: Seeds bio-primed with T. asperellum, T. harzianum, and T. asperellum + T. harzianum promoted the plant growth parameters and strengthening of physical barrier via lignification on the wall of vascular tissues. Seed primed with bioagents were used for exploring the molecular mechanism of defense response in pepper against anthracnose to assess the temporal expression of six defense genes in the Surajmukhi variety of Capsicum annuum. QRT-PCR demonstrated induction of defense responsive genes in chilli pepper bioprimed with Trichoderma spp. such as plant defensin 1.2 (CaPDF1.2), superoxide dismutase (SOD), ascorbate peroxidase (APx), guaiacol peroxidase (GPx), pathogenesis related proteins PR-2 and PR-5.
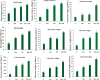
Results: The results showed that bioprimed seeds were assessed for T. asperellum, T. harzianum, and T. asperellum + T. harzianum-chilli root colonization interaction under in vivo conditions. The results of the scanning electron microscope revealed that T. asperellum, T. harzianum and T. asperellum + T. harzianum interact with chilli roots directly via the development of plant-Trichoderma interaction system. Seeds bio-primed with bioagents promoted the plant growth parameters, fresh and dry weight of shoot and root, plant height, leaf area index, number of leaves, stem diameter and strengthening of physical barrier via lignification on the wall of vascular tissues and expression of six defense related genes in pepper against anthracnose.
Conclusions: Application of T. asperellum and T. harzianum and in combination of treatments enhanced the plant growth. Further, as seeds bioprimed with T. asperellum, T. harzianum and in combination with treatment of T. asperellum + T. harzianum induced the strengthening of the cell wall by lignification and expression of six defense related genes CaPDF1.2, SOD, APx, GPx, PR-2 and PR-5 in pepper against C. truncatum. Our study contributed for better disease management through biopriming with T. asperellum, T. harzianum and T. asperellum + T. harzianum. The biopriming possess enormous potential to promote plant growth, modulate the physical barrier, and induced the defense related genes in chilli pepper against anthracnose.
Keywords: Bio-priming; Capsicum annuum; Defense related genes; Electron microscopy; Growth promotion; Lignification; Real-time PCR.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- FAOSTAT, 2020. Available at: http://faostat.fao.org/faostat/collections/subset/agriculture.
-
- Dubey MK, Zehra A, Aamir M, Yadav M, Samal S, Upadhyay RS. Isolation, identification, carbon utilization profile and control of Pythium graminicola, the causal agent of chilli damping-off. J Phytopathol. 2020;168(2):88–102. 10.1111/jph.12872.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

