Impaired dopamine release in Parkinson's disease
- PMID: 36864664
- PMCID: PMC10393405
- DOI: 10.1093/brain/awad064
Impaired dopamine release in Parkinson's disease
Abstract
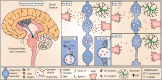
Parkinson's disease is the second most common neurodegenerative disease and yet the early pathophysiological events of the condition and sequences of dysfunction remain unclear. The loss of dopaminergic neurons and reduced levels of striatal dopamine are descriptions used interchangeably as underlying the motor deficits in Parkinson's disease. However, decades of research suggest that dopamine release deficits in Parkinson's disease do not occur only after cell death, but that there is dysfunction or dysregulation of axonal dopamine release before cell loss. Here we review the evidence for dopamine release deficits prior to neurodegeneration in Parkinson's disease, drawn from a large and emerging range of Parkinson's disease models, and the mechanisms by which these release deficits occur. The evidence indicates that impaired dopamine release can result from disruption to a diverse range of Parkinson's disease-associated genetic and molecular disturbances, and can be considered as a potential pathophysiological hallmark of Parkinson's disease.
Keywords: Parkinson’s disease; dopamine release; neurodegeneration.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures


References
-
- Zhang PL, Chen Y, Zhang CH, Wang YX, Fernandez-Funez P. Genetics of Parkinson's disease and related disorders. J Med Genet. 2018;55:73–80. - PubMed
-
- Dauer W, Przedborski S. Parkinson's disease: Mechanisms and models. Neuron. 2003;39:889–909. - PubMed
-
- Fearnley JM, Lees AJ. Ageing and Parkinson's disease: Substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–2301. - PubMed
-
- Correia Guedes L, Mestre T, Outeiro TF, Ferreira JJ. Are genetic and idiopathic forms of Parkinson's disease the same disease? J Neurochem. 2020;152:515–522. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

