The BISTIM study: a randomized controlled trial comparing dual ovarian stimulation (duostim) with two conventional ovarian stimulations in poor ovarian responders undergoing IVF
- PMID: 36864699
- PMCID: PMC10152167
- DOI: 10.1093/humrep/dead038
The BISTIM study: a randomized controlled trial comparing dual ovarian stimulation (duostim) with two conventional ovarian stimulations in poor ovarian responders undergoing IVF
Abstract
Study question: Is the total number of oocytes retrieved with dual ovarian stimulation in the same cycle (duostim) higher than with two consecutive antagonist cycles in poor responders?
Summary answer: Based on the number of total and mature oocytes retrieved in women with poor ovarian response (POR), there is no benefit of duostim versus two consecutive antagonist cycles.
What is known already: Recent studies have shown the ability to obtain oocytes with equivalent quality from the follicular and the luteal phase, and a higher number of oocytes within one cycle when using duostim. If during follicular stimulation smaller follicles are sensitized and recruited, this may increase the number of follicles selected in the consecutive luteal phase stimulation, as shown in non-randomized controlled trials (RCT). This could be particularly relevant for women with POR.
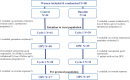
Study design, size, duration: This is a multicentre, open-labelled RCT, performed in four IVF centres from September 2018 to March 2021. The primary outcome was the number of oocytes retrieved over the two cycles. The primary objective was to demonstrate in women with POR that two ovarian stimulations within the same cycle (first in the follicular phase, followed by a second in the luteal phase) led to the retrieval of 1.5 (2) more oocytes than the cumulative number of oocytes from two consecutive conventional stimulations with an antagonist protocol. In a superiority hypothesis, with power 0.8 alpha-risk 0.05 and a 35% cancellation rate, 44 patients were needed in each group. Patients were randomized by computer allocation.
Participants/materials, setting, methods: Eighty-eight women with POR, defined using adjusted Bologna criteria (antral follicle count ≤5 and/or anti-Müllerian hormone ≤1.2 ng/ml) were randomized, 44 in the duostim group and 44 in the conventional (control) group. HMG 300 IU/day with flexible antagonist protocol was used for ovarian stimulation, except in luteal phase stimulation of the duostim group. In the duostim group, oocytes were pooled and inseminated after the second retrieval, with a freeze-all protocol. Fresh transfers were performed in the control group, frozen embryo transfers were performed in both control and duostim groups in natural cycles. Data underwent intention-to-treat and per-protocol analyses.
Main results and the role of chance: There was no difference between the groups regarding demographics, ovarian reserve markers, and stimulation parameters. The mean (SD) cumulative number of oocytes retrieved from two ovarian stimulations was not statistically different between the control and duostim groups, respectively, 4.6 (3.4) and 5.0 (3.4) [mean difference (MD) [95% CI] +0.4 [-1.1; 1.9], P = 0.56]. The mean cumulative numbersof mature oocytes and total embryos obtained were not significantly different between groups. The total number of embryos transferred by patient was significantly higher in the control group 1.5 (1.1) versus the duostim group 0.9 (1.1) (P = 0.03). After two cumulative cycles, 78% of women in the control group and 53.8% in the duostim group had at least one embryo transfer (P = 0.02). There was no statistical difference in the mean number of total and mature oocytes retrieved per cycle comparing Cycle 1 versus Cycle 2, both in control and duostim groups. The time to the second oocyte retrieval was significantly longer in controls, at 2.8 (1.3) months compared to 0.3 (0.5) months in the duostim group (P < 0.001). The implantation rate was similar between groups. The cumulative live birth rate was not statistically different, comparing controls versus the duostim group, 34.1% versus 17.9%, respectively (P = 0.08). The time to transfer resulting in an ongoing pregnancy did not differ in controls 1.7 (1.5) months versus the duostim group, 3.0 (1.6) (P = 0.08). No serious adverse events were reported.
Limitations, reasons for caution: The RCT was impacted by the coronavirus disease 2019 pandemic and the halt in IVF activities for 10 weeks. Delays were recalculated to exclude this period; however, one woman in the duostim group could not have the luteal stimulation. We also faced unexpected good ovarian responses and pregnancies after the first oocyte retrieval in both groups, with a higher incidence in the control group. However, our hypothesis was based on 1.5 more oocytes in the luteal than the follicular phase in the duostim group, and the number of patients to treat was reached in this group (N = 28). This study was only powered for cumulative number of oocytes retrieved.
Wider implications of the findings: This is the first RCT comparing the outcome of two consecutive cycles, either in the same menstrual cycle or in two consecutive menstrual cycles. In routine practice, the benefit of duostim in patients with POR regarding fresh embryo transfer is not confirmed in this RCT: first, because this study demonstrates no improvement in the number of oocytes retrieved in the luteal phase after follicular phase stimulation, in contrast to previous non-randomized studies, and second, because the freeze-all strategy avoids a pregnancy with fresh embryo transfer after the first cycle. However, duostim appears to be safe for women. In duostim, the two consecutive processes of freezing/thawing are mandatory and increase the risk of wastage of oocytes/embryos. The only benefit of duostim is to shorten the time to a second retrieval by 2 weeks if accumulation of oocytes/embryos is needed.
Study funding/competing interests: This is an investigator-initiated study supported by a research Grant from IBSA Pharma. N.M. declares grants paid to their institution from MSD (Organon France); consulting fees from MSD (Organon France), Ferring, and Merck KGaA; honoraria from Merck KGaA, General Electrics, Genevrier (IBSA Pharma), and Theramex; support for travel and meetings from Theramex, Merck KGaG, and Gedeon Richter; and equipment paid to their institution from Goodlife Pharma. I.A. declares honoraria from GISKIT and support for travel and meetings from GISKIT. G.P.-B. declares Consulting fees from Ferring and Merck KGaA; honoraria from Theramex, Gedeon Richter, and Ferring; payment for expert testimony from Ferring, Merck KGaA, and Gedeon Richter; and support for travel and meetings from Ferring, Theramex, and Gedeon Richter. N.C. declares grants from IBSA pharma, Merck KGaA, Ferring, and Gedeon Richter; support for travel and meetings from IBSA pharma, Merck KGaG, MSD (Organon France), Gedeon Richter, and Theramex; and participation on advisory board from Merck KGaA. E.D. declares support for travel and meetings from IBSA pharma, Merck KGaG, MSD (Organon France), Ferring, Gedeon Richter, Theramex, and General Electrics. C.P.-V. declares support for travel and meetings from IBSA Pharma, Merck KGaA, Ferring, Gedeon Richter, and Theramex. M.Pi. declares support for travel and meetings from Ferring, Gedeon Richetr, and Merck KGaA. M.Pa. declares honoraria from Merck KGaA, Theramex, and Gedeon Richter; support for travel and meetings from Merck KGaA, IBSA Pharma, Theramex, Ferring, Gedeon Richter, and MSD (Organon France). H.B.-G. declares honoraria from Merck KGaA, and Gedeon Richter and support for travel and meetings from Ferring, Merck KGaA, IBSA Pharma, MSD (Organon France), Theramex, and Gedeon Richter. S.G. and M.B. have nothing to declare.
Trial registration number: Registration number EudraCT: 2017-003223-30. ClinicalTrials.gov identifier: NCT03803228.
Trial registration date: EudraCT: 28 July 2017. ClinicalTrials.gov: 14 January 2019.
Date of first patient’s enrolment: 3 September 2018.
Keywords: IVF; dual ovarian stimulation; duostim; luteal phase stimulation; oocyte accumulation; poor ovarian reserve; poor ovarian responder.
© The Author(s) 2023. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Conflict of interest statement
This is an investigator-initiated study supported by a research Grant from IBSA Pharma. N.M. declares grants paid to their institution from MSD (Organon France); consulting fees from MSD (Organon France), Ferring, and Merck KGaA; honoraria from Merck KGaA, General Electrics, Genevrier (IBSA Pharma), and Theramex; support for travel and meetings from Theramex, Merck KGaG, and Gedeon Richter; and equipment paid to their institution from Goodlife Pharma. I.A. declares honoraria from GISKIT and support for travel and meetings from GISKIT. G.P.-B. declares Consulting fees from Ferring and Merck KGaA; honoraria from Theramex, Gedeon Richter, and Ferring; payment for expert testimony from Ferring, Merck KGaA, and Gedeon Richter; and support for travel and meetings from Ferring, Theramex, and Gedeon Richter. N.C. declares grants from IBSA pharma, Merck KGaA, Ferring, and Gedeon Richter; support for travel and meetings from IBSA pharma, Merck KGaG, MSD (Organon France), Gedeon Richter, and Theramex; and participation on advisory board from Merck KGaA. E.D. declares support for travel and meetings from IBSA pharma, Merck KGaG, MSD (Organon France), Ferring, Gedeon Richter, Theramex, and General Electrics. C.P.-V. declares support for travel and meetings from IBSA Pharma, Merck KGaA, Ferring, Gedeon Richter, and Theramex. M.Pi. declares support for travel and meetings from Ferring, Gedeon Richetr, and Merck KGaA. M.Pa. declares honoraria from Merck KGaA, Theramex, and Gedeon Richter; support for travel and meetings from Merck KGaA, IBSA Pharma, Theramex, Ferring, Gedeon Richter, and MSD (Organon France). H.B.-G. declares honoraria from Merck KGaA, and Gedeon Richter and support for travel and meetings from Ferring, Merck KGaA, IBSA Pharma, MSD (Organon France), Theramex, and Gedeon Richter. S.G. and M.B. have nothing to declare.
N.M. declares grants paid to their institution from MSD (Organon France); consulting fees from MSD (Organon France), Ferring, and Merck KGaA; honoraria from Merck KGaA, General Electrics, Genevrier (IBSA Pharma), and Theramex; support for travel and meetings from Theramex, Merck KGaG, and Gedeon Richter; and equipment paid to their institution from Goodlife Pharma; IA declares honoraria from GISKIT; and support for travel and meetings from GISKIT. G.P.-B. declares consulting fees from Ferring and Merck KGaA; honoraria from Theramex, Gedeon Richter, and Ferring; payment for expert testimony from Ferring, Merck KGaA, and Gedeon Richter; and support for travel and meetings from Ferring, Theramex, and Gedeon Richter. N.C. declares grants from IBSA pharma, Merck KGaA, Ferring, and Gedeon Richter; support for travel and meetings from IBSA pharma, Merck KGaG, MSD (Organon France), Gedeon Richter, and Theramex; and participation on advisory board from Merck KGaA. E.D. declares support for travel and meetings from IBSA pharma, Merck KGaG, MSD (Organon France), Ferring, Gedeon Richter, Theramex, and General Electrics. C.P.-V. declares support for travel and meetings from IBSA Pharma, Merck KGaA, Ferring, Gedeon Richter, and Theramex. M.Pi. declares support for travel and meetings from Ferring, Gedeon Richetr, and Merck KGaA. M.Pa, declares honoraria from Merck KGaA, Theramex, and Gedeon Richter; support for travel and meetings from Merck KGaA, IBSA Pharma, Theramex, Ferring, Gedeon Richter, and MSD (Organon France). H.B.-G. declares honoraria from Merck KGaA and Gedeon Richter; support for travel and meetings from Ferring, Merck KGaA, IBSA Pharma, MSD (Organon France), Theramex, and Gedeon Richter. S.G. and M.B. have nothing to declare.
Figures

Comment in
-
Low-quality evidence from a randomized controlled trial due to an inappropriate IVF setting to challenge Dual Stimulation strategy.Hum Reprod. 2023 Aug 1;38(8):1645-1647. doi: 10.1093/humrep/dead108. Hum Reprod. 2023. PMID: 37279888 No abstract available.
References
-
- Baerwald AR, Adams GP, Pierson RA.. Characterization of ovarian follicular wave dynamics in women. Biol Reprod 2003a;69:1023–1031. - PubMed
-
- Baerwald AR, Adams GP, Pierson RA.. A new model for ovarian follicular development during the human menstrual cycle. Fertil Steril 2003b;80:116–122. - PubMed
-
- Beckers NGM, Macklon NS, Eijkemans MJ, Ludwig M, Felberbaum RE, Diedrich K, Bustion S, Loumaye E, Fauser BCJM.. Nonsupplemented luteal phase characteristics after the administration of recombinant human chorionic gonadotropin, recombinant luteinizing hormone, or gonadotropin-releasing hormone (GnRH) agonist to induce final oocyte maturation in in vitro fertilization patients after ovarian stimulation with recombinant follicle-stimulating hormone and GnRH antagonist cotreatment. J Clin Endocrinol Metab 2003;88:4186–4192. - PubMed
-
- Bourdon M, Santulli P, Maignien C, Pocate-Cheriet K, Marcellin L, Chen Y, Chapron C.. The ovarian response after follicular versus luteal phase stimulation with a double stimulation strategy. Reprod Sci 2020;27:204–210. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

